Med Micro mizzou 3200 final
1/191
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
192 Terms
viruses cannot___ or ___ independent of host cel
generate energy or produce proteins
viruses do not have the genetic capability to multiply by
division
Viruses genome??
RNA OR DNA, NOT both
single stranded or double stranded
viruses have a naked ____ with attached____
capsid or envelope, proteins
virion
the infectious particle consisting of the envelope(some viruses), capsid and the interior core (genome +/-accessory viral proteins)
capsid
the outer protein shell that protects the interior core containing the genome and other proteins
genome
genetic material of the virus, either DNA or RNA
reduces transmission
-high rates of replication are often very damaging to host tissues which can kill the host
-high rates of replication are more likely to stimulate an immune response
increases transmission
genome/virion stability within host cells
tissue tropism
range of tissue types that a virus can infect
hit and run viral strategy
(small pox)
-viral production very high
-exposure relatively short
-large number of virions increases the likelihood of transmission (short-term)
Slow and low
(HEP. C)
-viral production very low
-exposure relatively long
-Immune evasion strategy
latency with occasional reemergence
(herpes simplex virus)
-viral production is moderate
-exposure is life long-viral genome integration
-transmission is relatively targeted
Icosahedral capsids
radial symmetry, based on polyhedron with 20 identical triangular faces
ex- herpes simplex virus (HSV)
filamentous capsids
helical symmetry, helical tube around the genome, which is wound helically within the tube
length can extend to 50x its width, generating flexible filament
ex- tobacco mosaic virus, Ebola virus
bullet shaped capsid
rabies virus
amorphous capsid
pox viruses
pleomorphic capsid
ebola virus
Avian Leukosis Virus (ALV)
small RNA retrovirus containing 3 genes--gag, pol, env
form 9 functional products
pandoravirus salinus
largest known virus to date
antigenic drift
virions no longer recognized by neutralizing antibodies
generates new strains of virus that can cause serious disease-classic ex is influenza virus
viral evolution
1. host community- evolve to preferentially infect different host species
2. viral species population- strains evolve that vary infectivity and virulence (ex- HHV1 and HSV2 cause similar diseases and HHV3 and HHV5 cause distinct diseases)
3. individual host organism- viruses evolve variants that resist therapeutic agents(HEP C & HIV)
Baltimore Model viral classification
-genome composition (DNA or RNA)
-whether it is single- or double-stranded
-If it is single stranded, whether the strand encodes proteins or requires synthesis or a complement that encodes proteins
7 baltimore groups
Group 1 of Baltimore model
Human Herpes Virus Double-stranded DNA is transcribed to mRNA
Group 2 of Baltimore model
Parovirus
single-stranded DNA
generates a double-stranded form within the host cell, which is transcribed to mRNA
Group 3 of Baltimore model
Rotavirus
double-stranded RNA
makes mRNA by using RNA-dependent RNA polymerase
Group 4 Baltimore model
Polio virus
single-stranded RNA(+)
makes complementary(-) strand which is transcribed to mRNA
Group 5 of Baltimore model
Influenza
Single-stranded RNA(-) is transcribed to mRNA
Group 6 of Baltimore model
HIV
single-stranded RNA(+) is reverse-transcribed to DNA, which is transcribed to mRNA
group 7 of Baltimore
HEP B
Double-stranded DNA is transcribed to mRNA which is reverse-transcribed to make viral genomes for packaging into virions
lytic replication
replication cycle usually results in death and lysis of host cell
stages:
attachment & entry
synthesis and assembly
exit and transmission
direct penetration
viral capsid of genome is translocated directly into cytoplasm
membrane fusion
viral envelope fuses w the PM releasing the nucleocapsid into the cytoplasm--> capsid breaks down
endocytosis
virion-receptor complex is endocytosed into cytoplasm
viral envelope fuses w the endosomal membrane releasing nucleocapsid---> capsid breaks down to release genome
DNA viruses typically enter where and why
nucleus- they require the host cell's DNA-dependent RNA polymerase
where do RNA viruses typically replicate?
cytoplasm
HP DNA Genome
-HPV gains access to the actively dividing cells of the epidermis basal layer
-virions are endocytosed by the basal cells (but no replication until basal cells start to differentiate into keratinocytes)
-as epithelial layers slough off, progeny virions are shed
-sometimes HPV virions become latent, persisting for months or years & the latent viral genomes may induce host cells to form abnormal growths, such as warts or cancers
viral pathogenesis in general derives from
-latency
-cell death/damage
-Immune-mediated damage
properties of true latency
maintenance
persistence
reversibility
episomal latency
use of episomes during latency (extra-chromosomal genetic material that may replicate autonomously) --herpes
proviral latency
occurs when the viral genome is integrated into the host chromosome(s)--retroviruses such as HIV-1
cell death (viral pathogenesis cytopathic effects)
can be virally-Induced lysis(adenovirus) or cellular apoptosis (poliovirus)
cell fusion (viral pathogenesis cytopathic effects)
certain viruses promote cell-cell fusion to generate, giant multinucleate cells (HIV,RSV)
malignant transformation (viral pathogenesis cytopathic effects)
certain viral infections can lead to cellular transformation ---cancer development
cytotoxic t cells - (viral pathogenesis immune mediated injury)
HAV & HBV both stimulate production of CTLs which kill infected hepatocytes--accounts for majority of damage to the liver
immune complex deposition (viral pathogenesis immune mediated injury)
HBV-antibody complexes can become deposited on capillary membranes--> immune mediated vasculitis
plaque assay
1. infect monolayer w virus
2. remove liquid medium
3. add gelatin medium
4. virus reproduces, host cells lyse...forming plaques
this can be used to estimate the number of infectious particles (plaque-forming units, pfu) in a sample
PCR
Direct detection of viral genomic material, high sensitivity can detect both DNA & RNA viruses, can multiplex. screen for multiple pathogens in a single sample
acute coryza (rhinitis) commonly assoc. w -
rhinoviruses, coronaviruses
influenza commonly assoc. w-
influenza viruses
Croup is commonly assoc. w -
parainfluenza viruses
Bronchiolitis commonly assoc. w-
RSV
Bronchopneumonia
influenza virus, RSV, Adenoviruses
influenza pathogenesis
1.direct cell lysis (primary) upper/lower respiratory tracts--edema/inflammation
2. role of immune response -primarily protective rather than pathogenic, induces virus- and type-specific immunity, virus-mediated suppression (NS1 protein)
major complications influenza virus infections
secondary bacterial pneumonia- (superinfection) -> death aka S. pneumonia, H. influenzae, S. aureus
classic signs and symptoms of severe influenza infection
rapid onset of symptoms
fever- over 38.5
early, nonproductive cough and headache
severe myalgia and fatigue
runny/stuffy nose
sore throat
seizures, night/cold sweats, no appetite, vomiting & diarrhea(cell breakdown products in body)
influenza A antigenic drift
due to gene shuffling and reassortment
requirements:
segmented genome (8RNA segs)
multiple HA & NA subtypes in environment
animal reservoir (wild aquatic birds)
susceptible species for both avian and human influenza to undergo recombination (swine)
infleunza treatment
neuraminidase inhibitors
-oseltamivir(tamiflu, oral)
-zanamivir (relenza, inhaled)
-peramivir (rapivab, IV)
common cold symptoms not related to flu
high fever is rare
headache is rare
myalgia is mild flu is severe
fatigue is mild and flu is sever and prolonged
Rhinoviruses
baltimore group 4
non-enveloped
non-segmented (+) single stranded RNA genome
at least 100 serotypes are known
sensitive to low pH (no GI infection)
optimal growth at 32 degrees- adaption to nasal cavity
rhino viral pathogenesis
minimal direct virus-Induced cell damage
primarily upper respiratory tract
immune response (histamine release) correlates w symptoms
responsible for COPD & asthma exacerbations
induces serotype-specific immunity
coronaviruses
baltimore group 4
pleomorphic, enveloped virus
non-segmented(+) ss-RNA genome
6 human serotypes known- 4 commonly infect humans causing common cold
compared to rhinoviruses-- longer incubation period and shorter symptoms
SARS-CoV & MERS-CoV= rare and life-threatening
Adenoviruses
baltimore group 1
non-enveloped
icosahedral nucleocapsid containing linear dsDNA
at least 57 serotypes
causes 3-5% of respiratory infections in children, less than 2% in adults
Clinical manifestations of adenoviruses
rarely cause serious illness of death
mostly just
common cold-mostly in young kids
pink eye
bladder inflammation or infection (cystitis)
acute respiratory disease (ARD)
sever acute respiratory syndrome (SARS)
emerged in china 775 deaths
SARS caused by the SARS-associated coronavirus (SARS-CoV)
insidious flu-like symptoms --> atypical pneumonia based on CXR
fever greater than 38 degrees C
20-30% respiratory failure, ventilator
lower GI- diarrhea
paramyxoviridae
visions are enveloped-spherical, filamentous or pleomorphic
genome is non-segmented, -ssRNA, 6-10 genes
Group 5
respiratory syncytial virus
parainfluenza viruses
mumps
measles
human metapneumovirus
Hendra, Nipah
Parainfluenza virus
infect respiratory epithelium, large airways
3 major envelope proteins
Haemagglutinin (H)-binds to cell surface
Neuraminidase (N)- release from cell surface
Fusion(F) protein-promotes membrane fusion
clinical manifestations of parainfluenza
5 mil hospitalizations in US each year under 5 years old
often promotes secondary bacterial infection (otitis media)
acute laryngo-tracheo bronchitis (croup), severe URI of infants (6-48 mos)
croup
mild URI initially
barking couch
hoarseness
sore throat
stridor (airway obstruction)
clinical diagnosis based on symptoms
chest xray reveals characteristic "steeple sign"
RSV- respiratory syncytial virus
classified into groups A&B by sera
cytolytic infections of bronchiole epithelium
primary infection of young kids
adults are at increased risk of bronchial asthma
RSV formation
envelope contains Fusion(F) protein- viral entry and syncytium formation
syncytial-giant, multinucleate cells
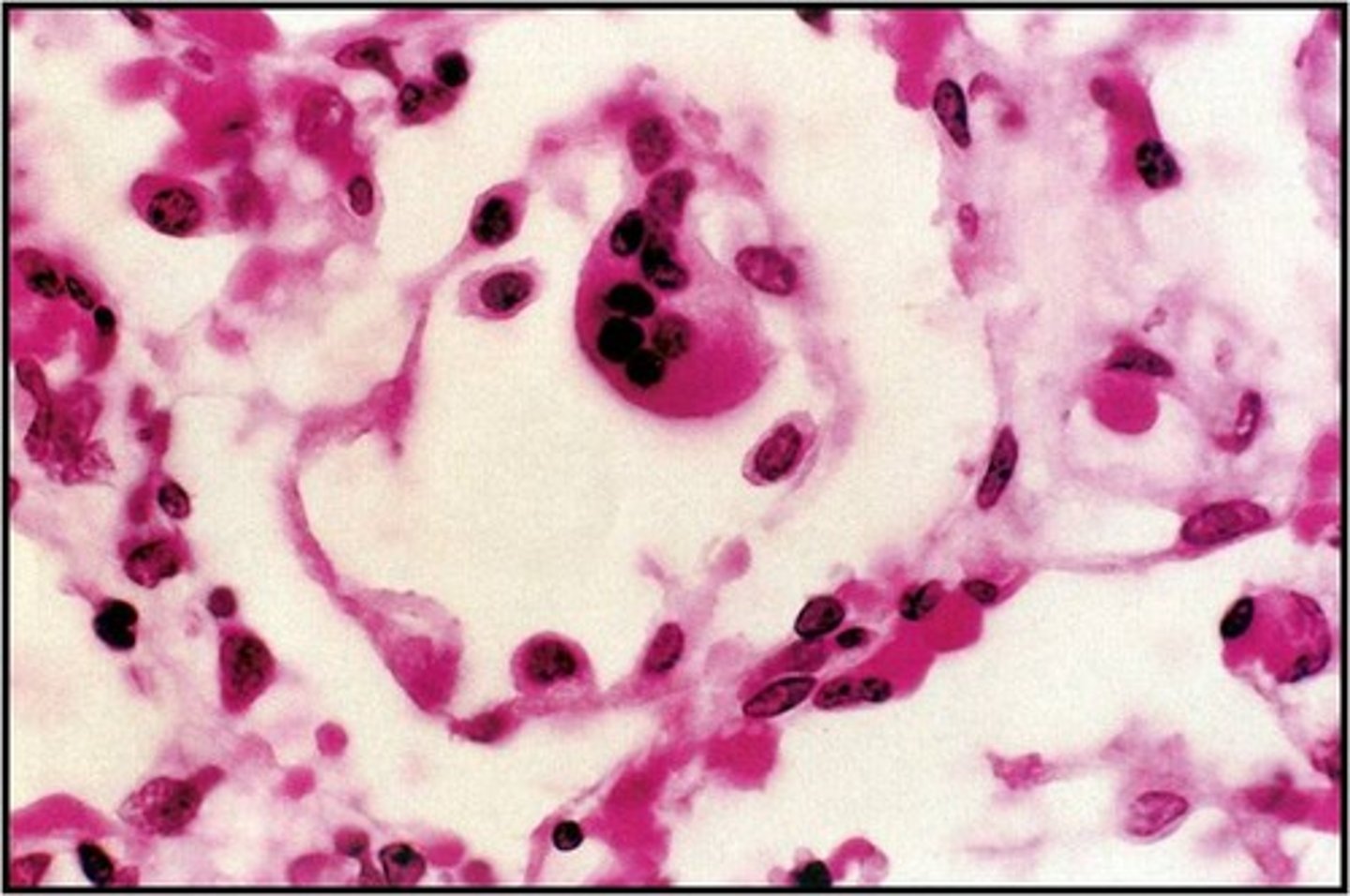
Bronchiolitis
URI
then cough, wheezing and difficulty breathing (dyspnea), chest x ray reveals hyperinflation
severe:
poor feeding, lethargy history of apnea, respiratory rate under 70/min, severe chest wall recession, cyanosis (blue coloring or skin and mucuous membranes due to poor o2)
pt.s may require 02, intubation or mechanical ventilation
viruses assoc. w gastroenteritis(stomach flu)
-rotavirus
-norovirus
-adenovirus types 40, 41, &52
-astrovirus
NOT: picornaviridae
Rotavirus
infants and children (~100& by age 5)
adults in US--> child care workers, elderly, travelers
Strain A- 90% of infection in humans
infects/damages mature enterocytes leading to severe malabsorption and loss of disaccharides(2ndary lactose intolerant) - osmotic type diarrhea
Gastroenteritis-mild to severe disease symptoms
watery, dark green, explosive diarrhea
vomiting, abdominal pain
low-grade fever
lasts 3-5 days can cause dehydration
Rotavirus Non Structural Protein 4 (NSP4)
first identified viral enterotoxin
stimulates enteric nerves--hyperperistalsis (overactivity of intestinal muscles)
stimulates Vagus nerve-- promotes vomiting
stimulates salt and water secretion -- secretory type diarrhea
dx- ELISA, PCR
rx- ORS
Norovirus
leading cause of food borne illness in US
transmitted
fecal-oral
person to person contact
aerosolization of vomited virus
classic scenarios for outbreak- cruise ships
pathology of norovirus
shortening of enterocyte villi
crypt cell damage
decreased digestive enzymes (lipase & disaccharidases)
damage to laminitis propria due to immune cell infiltration
diseases assoc. w poliovirus (enteroviruses)
paralytic poliomyelitis
diseases assoc. with coxsackle group A (enteroviruses)
hand, food and mouth disease
skin & mucosa membranes
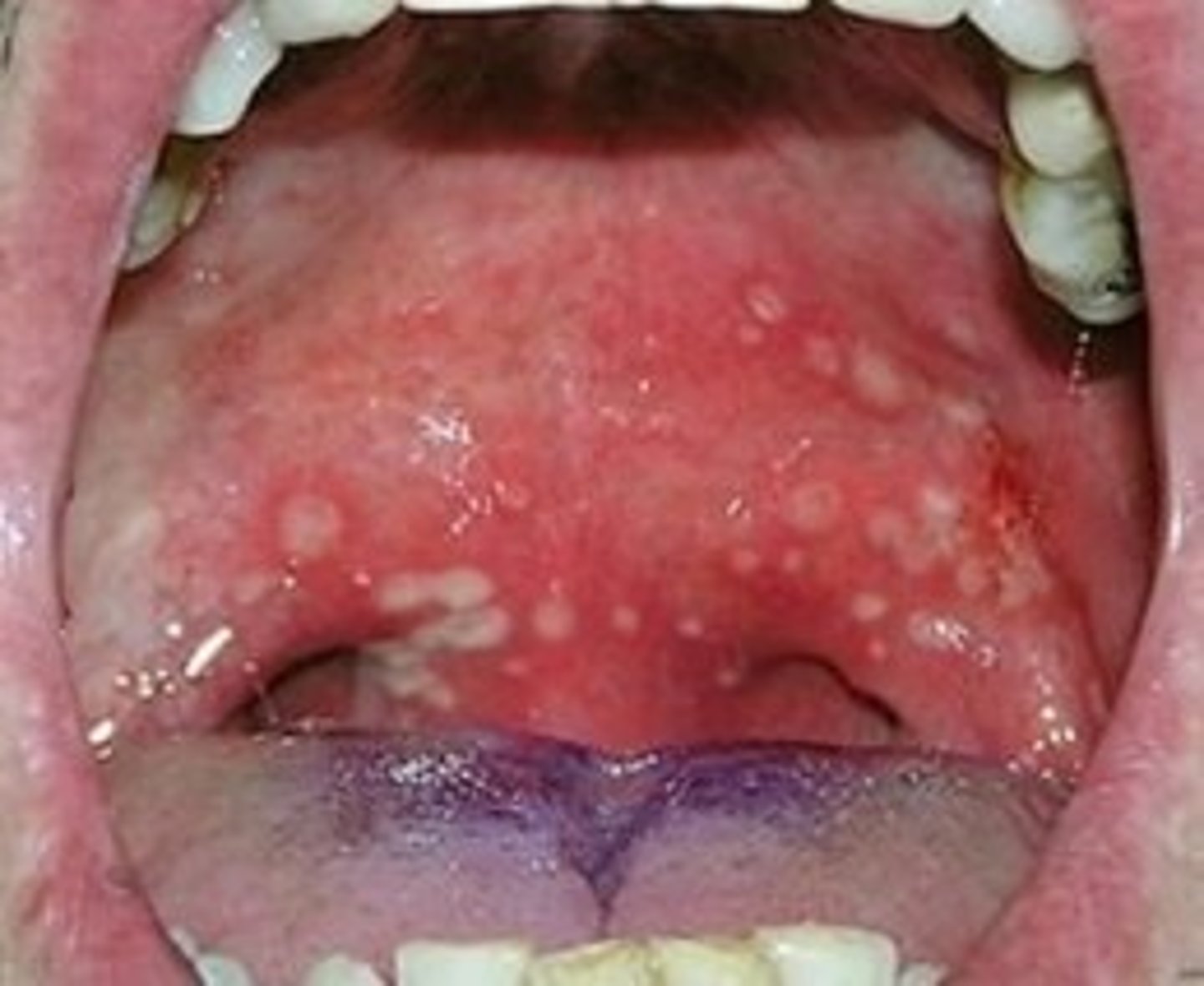
diseases assoc. with coxsackle group B (enteroviruses)
myopericarditis, pericarditis
heart, pleura, pancreas, and liver
diseases assoc. with echovirus (enteroviruses)
aseptic meningitis, rash, febrile illness, conjunctivitis, severe generalized neonatal disease
diseases assoc. with Enterovirus (enteroviruses)
polio-like illness, hemmorrhagic conjunctivitis (E70)
Polio virus
human specific pathogen
highly contagious (oral-oral, fecal-oral)
following ingestion--> virus multiples within GI mucosa--> transient viremia 75% of patients, asymptomatic
25% symptoms in 2-5 days
fever, sore throat, tiredness, nausea, headache, stomach pain
paralytic poliomyelitis
~1% infections, poliovirus invades and replicates within motor neurons
motor neuron death leads to flaccid paralysis-muscle atrophy over time
many cases result in only temp. paralysis
Hepatitis A (infectious hepatitis) transmission
fecal-oral - food handlers (raw fruits/veggies), raw shellfish, water supply
close personal contact- household contact, sexual contact, child day care centers
blood exposure (rare) - injecting drugs, transfusions
Clinical features of hep A
incubation period 3-4 wks
general symptoms- flu-like, fever/chills, body aches, fatigue, nausea/vomiting
jaundice by age group <14-0-50%
>14-70-80%
complications- relapsing, cholestatic hep., fulminant hep.
HAV diagnosis
HAV-IgM- 1-14 wks post infection
HAV-IgG- clearance of infection (high titers indicate protection)
ALT- liver enzyme (releases due to liver damage)
HEP B (serum hepatitis)
"Dane particle" = infectious form
Hep. B transmission
Sexual- predominant transmission route
Perinatal- mothers HBeAg positive--predominant in endemic populations like s.e Asia
Parenteral-needle stick in none immune, 30%.. rare-surgeon, dentist, medical equipment
HBV Pathogenesis
primary infection: viremia->liver->replicates in hepatocytes (not cytotoxic)
replication involves RNA intermediate (HBV reverse transcriptase)
HBV clinical features
-incubation period- 90 days
-general symptoms- poor health, loss of appetite, nausea, vomiting, aches, mild fever, dark urine
-chronic hepatitis
HBV Treatment
most adults clear infection spontaneously
-reverse transcriptase inhibitors (lamivudine, adefovir dipivoxil, entecavir, telbivudine, tenofovir) - all drugs active against HIV to some extent
HEP C
Most common chronic blood borne infection
causes 40% of chronic liver disease
incubation period 7-8 wks
75% are subclinical
symptoms mild and vague - acute liver failure
85% of adults have a chronic infection
CD4+ T cells normal range
450-1500
oral candidiasis is common in
HIV+ (10-40%) of pts.
AIDS (90%) of pts.
tests for HIV
HIV ELISA test
Western blot
Retroviruses
-contain two, noncovalently linked copies of the (+) ssRNA genome --> pseudodipliod
-nucleocapsid is surrounded by an envelope that contains spikes formed by a virus-encoded glycoprotein
-virion contains 10-50 copies of reverse transcriptase
-Integrase enzymes -- provirus integration
two types: primarily oncogenic and primarily immunosuppressive
HIV-1
is encountered globally and accounts for the majority of infections in North America and Europe
HIV-2
characterized by a longer asymptomatic stage and lower mortality , progression to AIDS does occur
largely restricted to Western Africa