Non-Polar side chains
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what are these amino acids made of
Carbon and hydrogen
Do they like water ?
no . their hydrophobic
Non-polar = no charge difference so because of this
their side chain doesnt attract to water
they dont accpet or donate protons. not acidic or basic
No hydrogen or ionic bonding so because of this
they dont have electronegative atoms O and N in side chains
cant form hydrogen bonds or ionic bonds.
Shape and size vary because of this …
come in different types rings,
hydrogen side chain.
Some are largerhave carbon chains
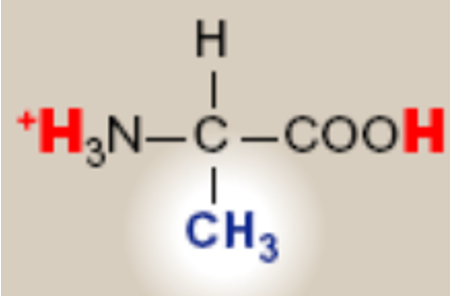
Alanine; Ala: A
saturated hydrocarbon R groups
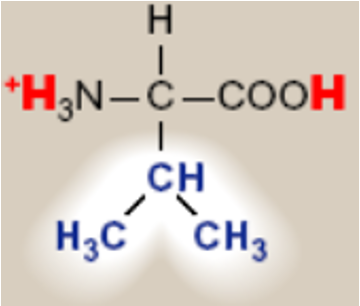
Valine; Val; V
saturated hydrocarbon R groups
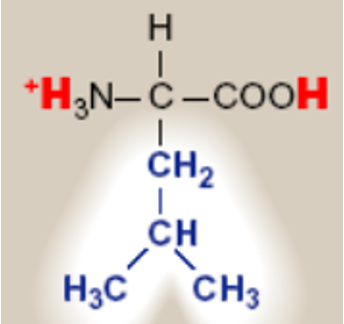
Leucine; Leu; L
saturated hydrocarbon R groups
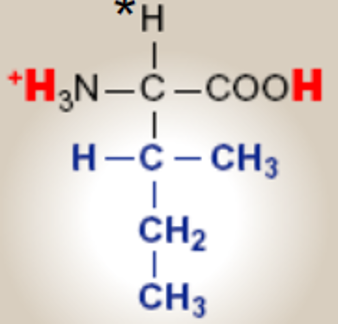
Isoleucine; Ile; I.
saturated hydrocarbon R groups
Non-polar Side Chains (Extra Details)
These amino acids still have hydrophobic (non-polar) side chains
but some have special features that affect protein structure.
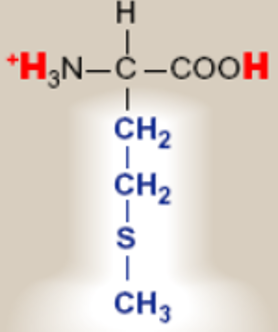
Methionine; Met; M
has a sulphur atom in the R group
Glycine; Gly; G
Glycine; Gly; G features
its R group is just a hydrogen (H).
its not chiral Because both side groups on the central carbon are hydrogens
small size makes it very flexible to polypeptides
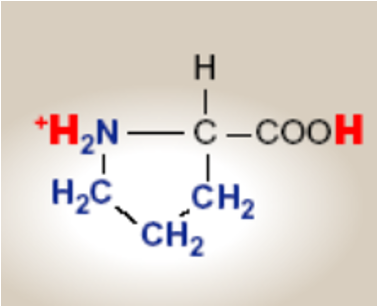
Proline; Pro; P
Proline; Pro; P features
Has a cyclic ring r group side chain, that connects back to its own amino group
forms imino group
Because its ring locks the structure, proline is stiff and restricts movement in the polypeptide chain.
Water and “Oily” (Hydrophobic) Amino Acids
Water is polar — it has a positive side and a negative side
Non-polar R groups (the side chains of some amino acids) are like oil — they don’t mix well with water.
call them hydrophobic,
What Happens in Proteins:
f the protein is in water (aqueous solution):
The hydrophobic (oily) R groups try to avoid water.
They stick together and hide inside the protein’s center.
The outside of the protein has polar (water-loving) amino acids that can interact with water.
f the protein is in a membrane (fatty environment):
The membrane is made of lipids (fats), which are also non-polar.
So here, the hydrophobic R groups face outward to interact with the fatty membrane.
The polar parts stay inside, away from the lipids.