Things I got wrong on my chemistry exams 1-4
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Which of the following would be electrically attracted to a proton?
a. proton b. neutron c. electron d. none of these
c. electron
If a person runs a marathon (26.2 miles) in exactly 2.00 hours, how fast did the person run the marathon in units of meters per second? 1 mile = 1609 meters
5.85 m/s
How many nanometers are in 5.0 cm? Express your answer in scientific notation.
5.0×10^7
A box has a volume of 5.1×104 cm3 What is the volume of the box in units of L?
51L
Calculate the atomic weight of an element that has three naturally occurring isotopes with the following masses and abundances.
mass 19.99 amu abundance 90.51%
mass 20.99 amu abundance 0.27%
mass 21.99 amu abundance 9.22%
20.1771 amu
Which of the following ions would be attracted to the hydrogen atoms on a water molecule?
a. Na+ b. Ca2+ c. Fe3+ d. none of these
d. none of these
Which of the following is a strong base?
a. HCl b. Ca(OH)2 c. NH3 d. none of these
b. Ca(OH)2
What is true about this reaction C3H7COOH (aq) ←→ C3H7COO-(aq) + H+
a. It represents a strong electrolyte dissolving in water
b. It represents the dissociation of a weak base
c. It represents the dissociation of a strong acid
d. It represents the dissociation of a weak acid
d. It represents the dissociation of a weak acid
An electrolyte is a substance that:
a. increases the concentration of of H+ (aq) in solution
b. increases the concentration of OH+ (aq) in solution
c. conducts electricity when dissolved in water
d. none of these
c. conducts electricity when dissolved in water
A compound with a molar mass of 78.1 g/mol is made only of the elements carbon and hydrogen. When the compound is completely combusted, 2.311 g of CO2 and 0.4729 g of H20 are produced. What is the molecular formula of the compound?
C6H6
What is the oxidation number on chromium in CrO4-
+6
How many milliliters of 0.350 M KMnO4 are required to completely react with 10.50 grams of FeCl2 (FW = 187.5 g/mol) according to the following reaction:
8H+(aq) + KMnO4(aq) + 5FeCl2(aq) → 5FeCl3(aq) + 2MnCl2 + 4H2O(l) + KCl(aq)
32 mL
A scuba diver blows out a bubble with a volume of 125 mL at the bottom of a lake where the temperature is 4.0 °C and the pressure is 1725 mmHg. What will the volume of the bubble be when it reaches the surface where the temperature is 30.0 °C and the pressure is 745 mmHg?
317 mL
A candy bar has 151 nutritional Calories. How many kilojoules of energy is this?
631.784 kj
How many grams of propane (C3H8 MW = 44,09 g/mol) would you need to burn to provide 1105 kJ of heat?
23.8 g
A photon of which color would have the lowest energy?
a. red (lambda = 650 nm) b. yellow (lambda = 580 nm) c. blue (lambda = 515 nm) d. all photons have the same energy
a. red (lambda = 650 nm)
Forming chemical bonds _____ energy, and breaking chemical bonds ______ energy.
a. requires, requires
b. require, releases
c. releases, requires
d. releases, releases
c. releases, requires
Which of the following covalent bonds between carbon atoms is the longest?
a. C-C b. C 2 lines C c. C three lines C d. they all have the same length
a. C-C
What element has the electronic configuration [Kr] 5s24d105p4
Te
Which of the following atoms has the lowest ionization energy: O, S, Po
Po
How many core electrons does an atom of phosphorus have?
10
Write the orbital diagram (with arrows) for an atom of nickel in the space below. You may use noble gas notation.
Orbital diagram for nickel
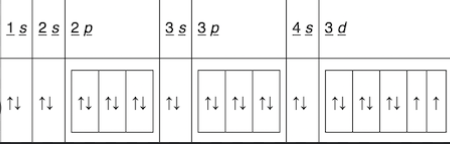
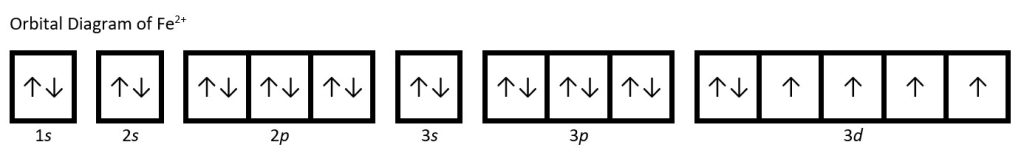
Write the orbital diagram (with arrows) for an atom of Fe2+ in the space below, You may use noble gas notation.
Orbital diagram for Fe2+
What is the formal charge on the boron and oxygen atoms in the borate ion, BO33-
B: O:
B: 0 O: -1