Organic Chemistry A-level OCR
1/140
Earn XP
Description and Tags
Currently only ALL of AS content
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
141 Terms
Key features of a homologous series.
Same functional group: this is a specific group of atoms within a molecule that define key chemical characteristics.
Formula: all members have the same general formula. This can be used to predict the molecular formula of any member in the series.
Difference: each subsequent compound in the series only differs by a CH2 unit, this allows you to predict the physical properties within the same series
What is a functional group?
A functional group is a group of atoms that are responsible for the characteristic reactions of a compound
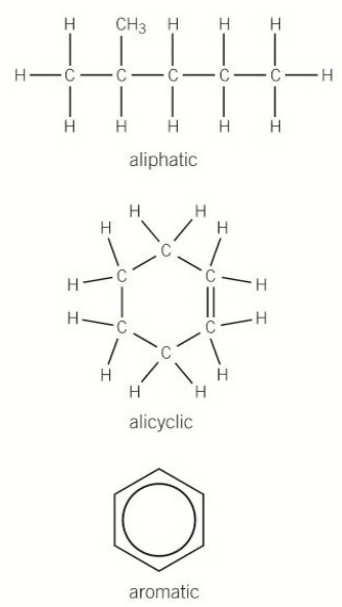
General Classification based on reactivity due to the type of bonds between the carbons
Saturated compounds have only single bonds between carbon atoms, like in alkanes.
Unsaturated compounds contain double or triple bonds (C=C, C≡C), or are aromatic.
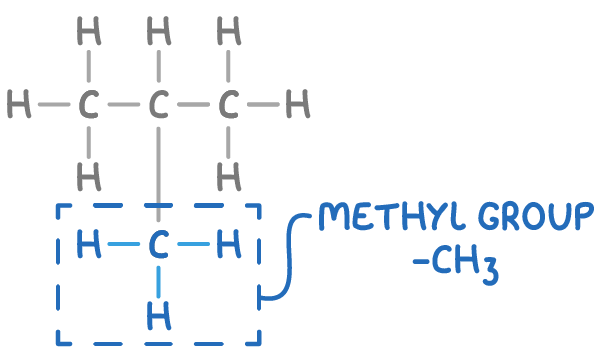
What is an alkyl group?
Finally, an alkyl group is a fragment of a molecule, a carbon chain with the general formula CnH2n+1.
For example, the methyl group (-CH3) is the smallest alkyl group, circled in the molecule below.
3 broad types of structures carbon can form

3 Things a carbon can do when bonding
4 single bonds
1 double bond & 2 single bonds
1 triple bond & 1 single bond
2 double bonds are just not stable enough
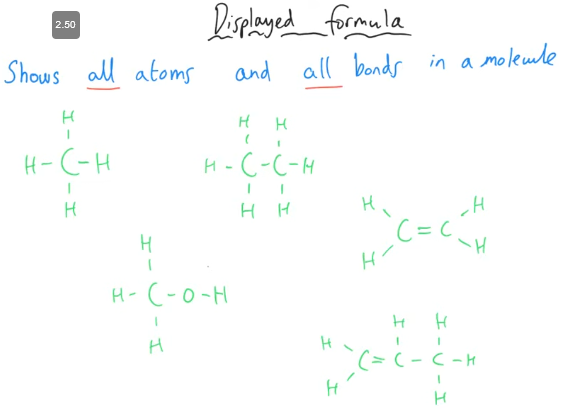
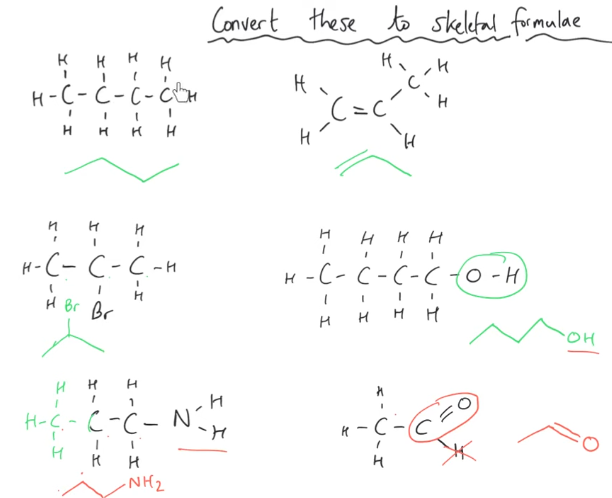
What is displayed formula?

What is structural formula?
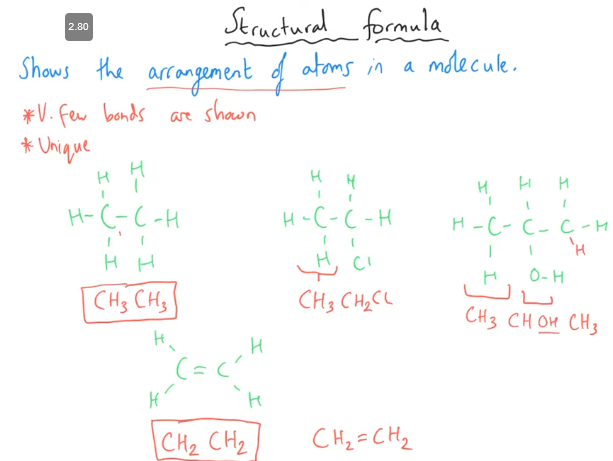
You are aiming to show as much info as possible with letters, so do not merge stuff together.
E.g. CHOH should not be CH2O, as this will remove the info about an OH group.
What is skeletal formula
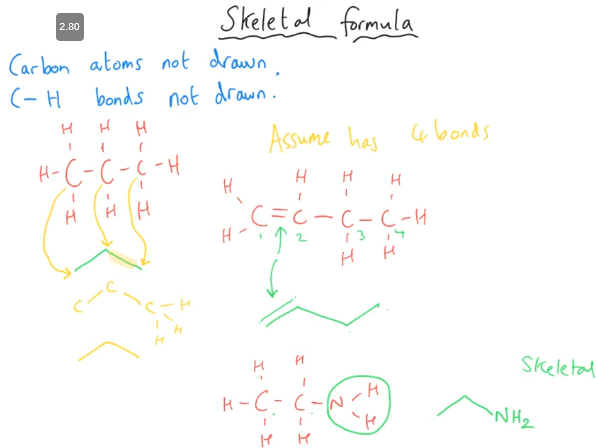
Each vertex is a Carbon
Carbon to Carbon, or Carbon to Hydrogen is never shown
Tell me about alkanes

Tell me about Halogenalkanes

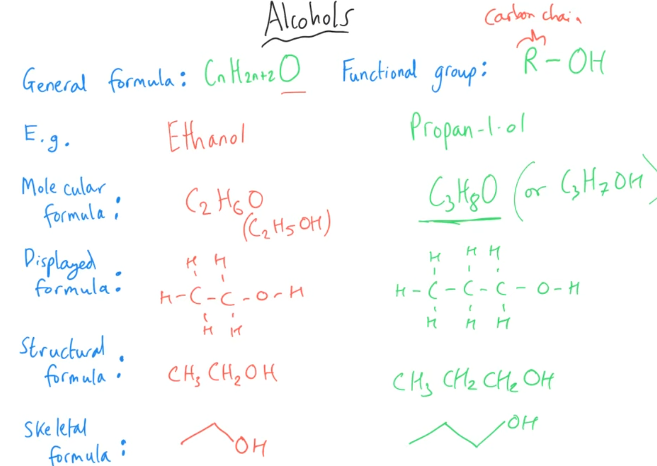
Tell me about Alcohols


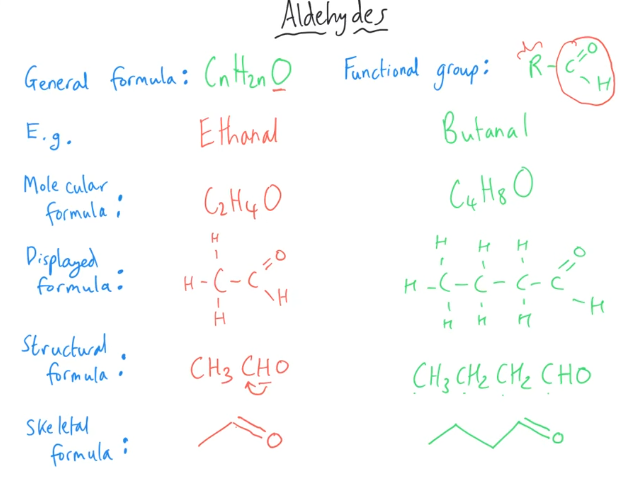
Tell me about Aldehydes

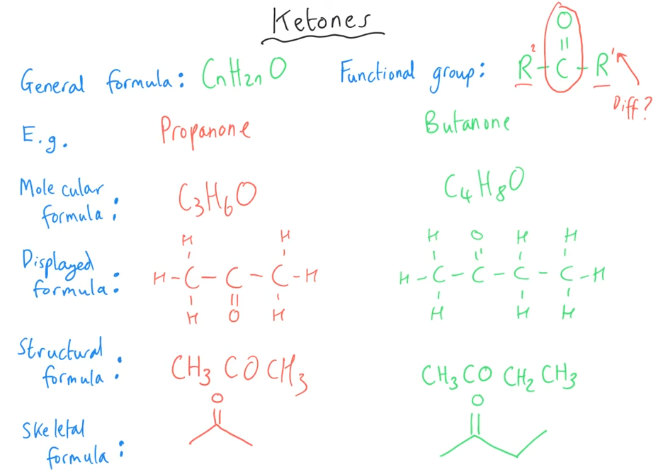
Tell me about Ketones

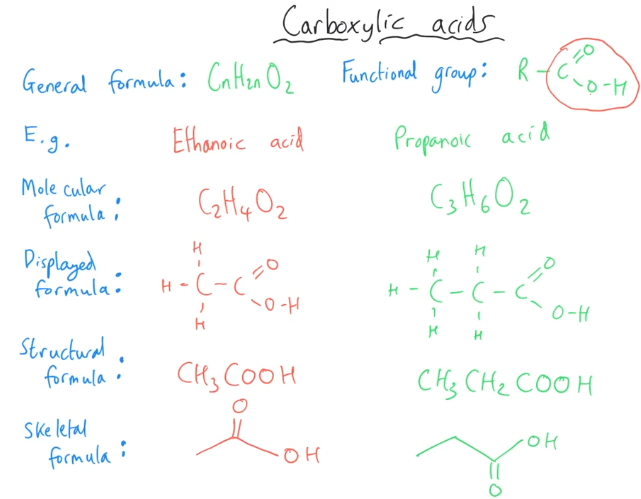
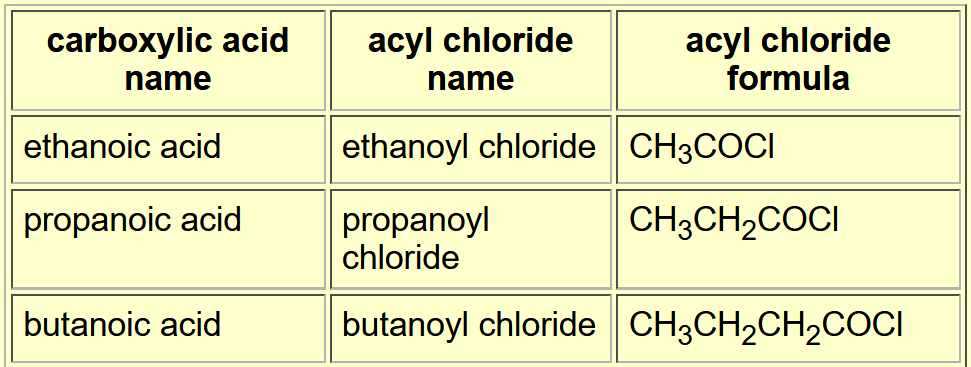
Tell me about Carboxylic Acids


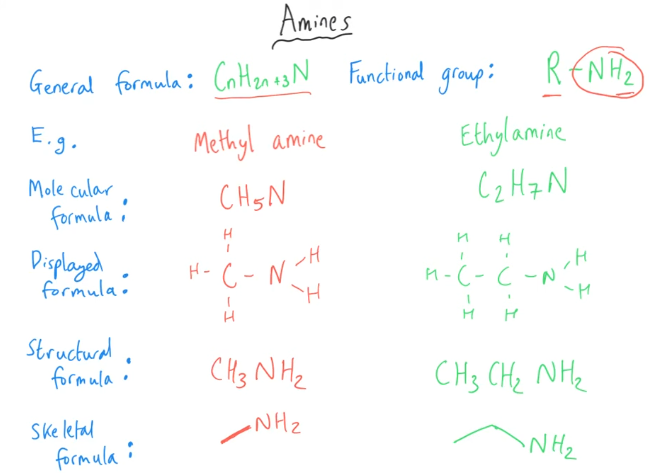
Tell me about amines


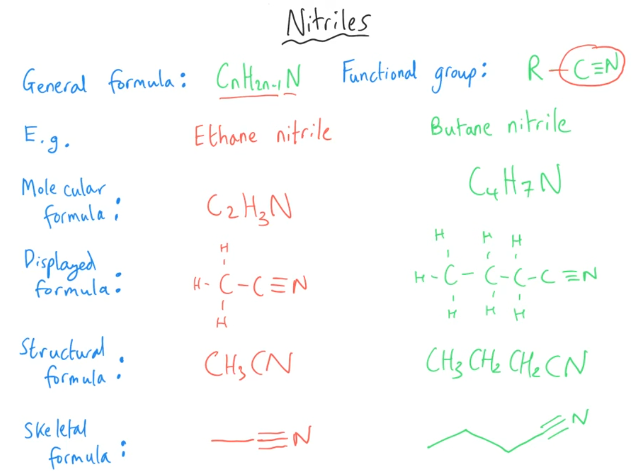
Tell me about Nitriles


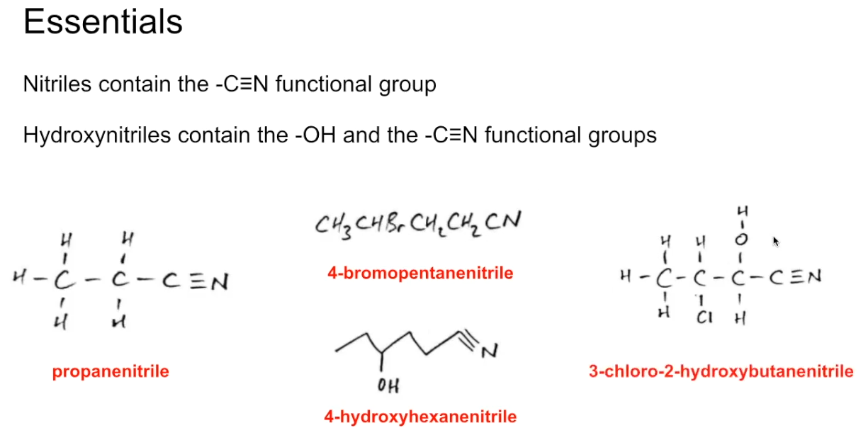
What are the other types of nitriles?

Tell me about acyl group
Specific example.
How does the naming work?
They are similar to carboxylic acids.

But they are described as acid derivatives, as they are when the OH has been replaced by a chloride.
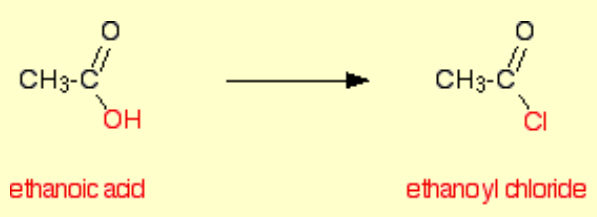
An acyl chloride is when the OH is replaced by a chloride, such as ethanoyl chloride
They are very reactive, and cannot be dissolved as they will react with the water itself.
They are a colourless fuming liquid.

Tell me about esters.


Suffix is -oate
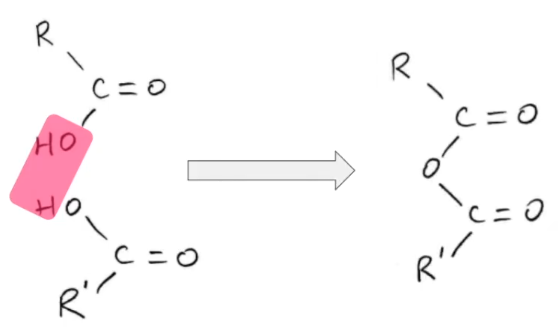
Tell me about anhydrides
Two carboxylic acids minus a water molecule
Tell me about Alkynes
They have at least one triple carbon-to-carbon bond
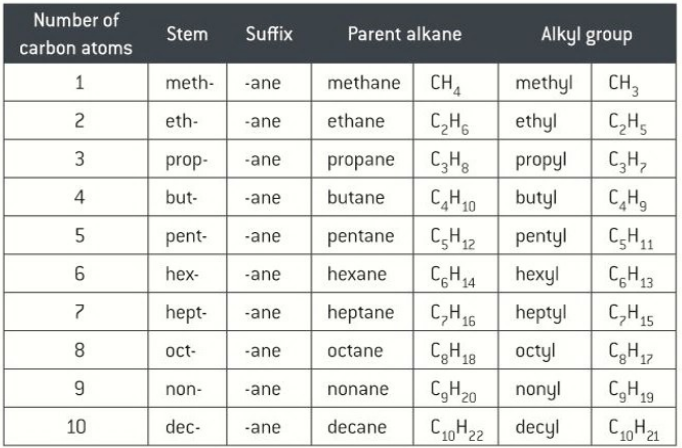
Number prefixes for Organic Chemistry
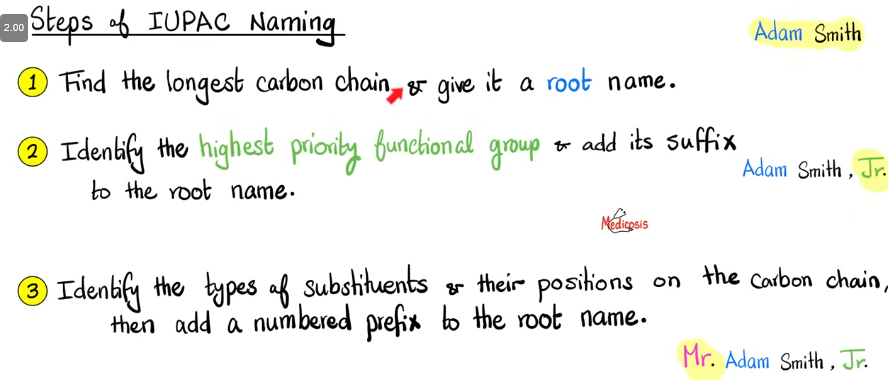
How to write elements with the right IUPAC name?

Suffix for Organic chemistry

Branches of from main carbon line, numerical prefixes
di - 2
tri - 3
tetra - 4
Names with ethers
1: methoxy
2: ethoxy
3: propoxy
4: butoxy
Alkene vs Alkyne in IUPAC naming
In IUPAC nomenclature, when both a double bond (alkene) and a triple bond (alkyne) are present in the same molecule, the parent chain should include both if possible.
The chain is numbered from the end nearest the first multiple bond (whether double or triple).
If both could be at the same position (i.e., both could be carbon 1), then the alkene (double bond) gets the lower number — it takes priority in numbering, not in naming or functional priority.
However, if they’re at different positions, the one that gives the lowest possible locant overall is preferred, and that can be the alkyne.
✅ Summary rule:
When deciding numbering: the first multiple bond (either) gets the lowest possible number.
When there’s a tie (both could start at carbon 1): the alkene wins.
When naming: if both are present, the compound is named as an “en-yne”, and the “en” (alkene) part is written before the “yne” (alkyne).
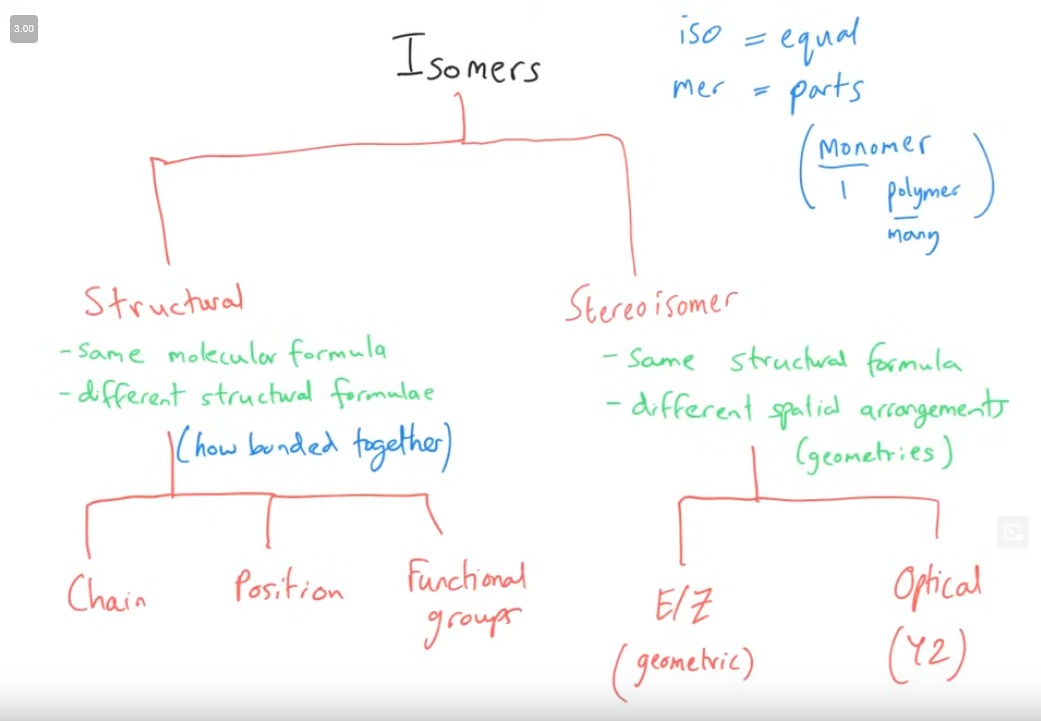
Tell me about Isomers
Tell me about the 1st structural isomer
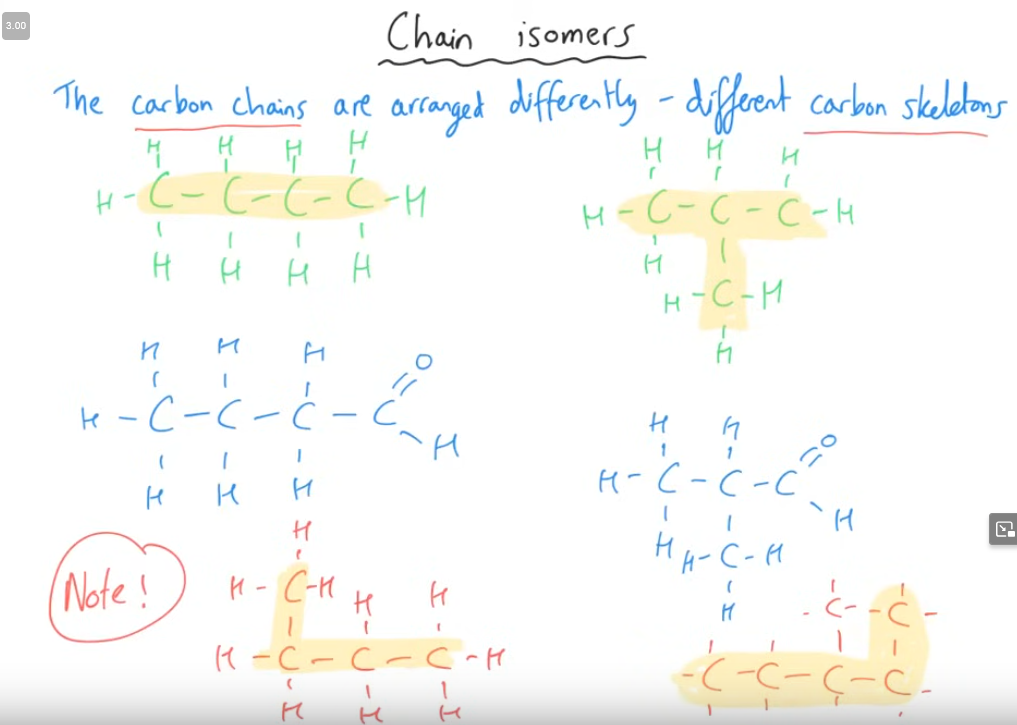
The bottom shows a butane on the left, and a 3-methyl pentane on the right.
Tell me about the 2nd structural isomer
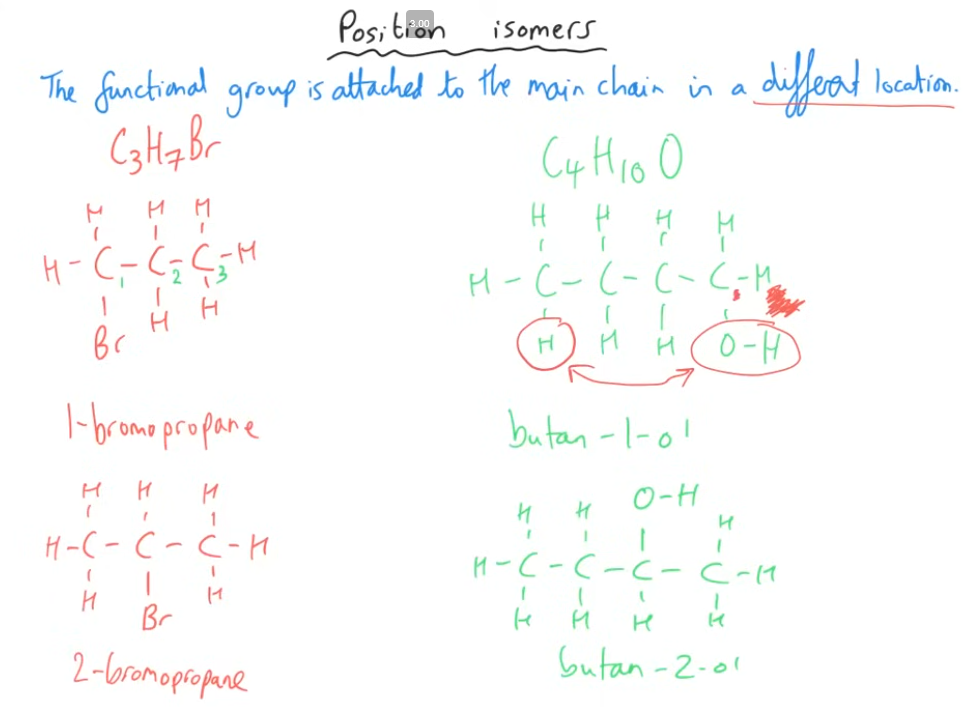
Note: for it to be a positional isomer, the position of the functional group must change, it cannot just be on the same carbon
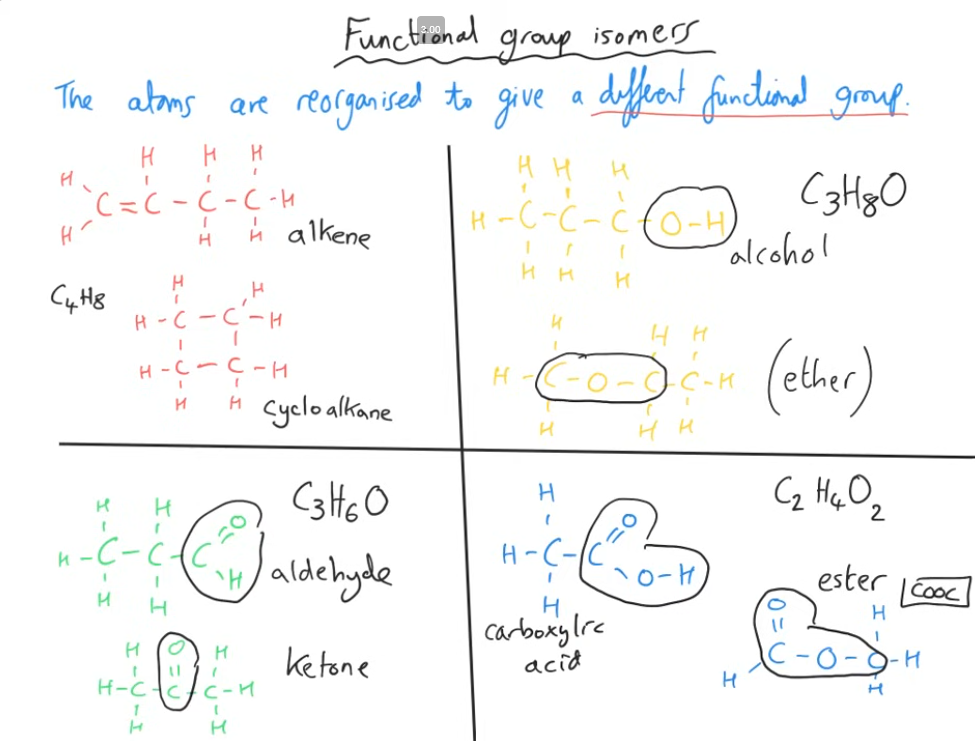
Tell me about the 3rd type of structural isomer, and 4 common examples
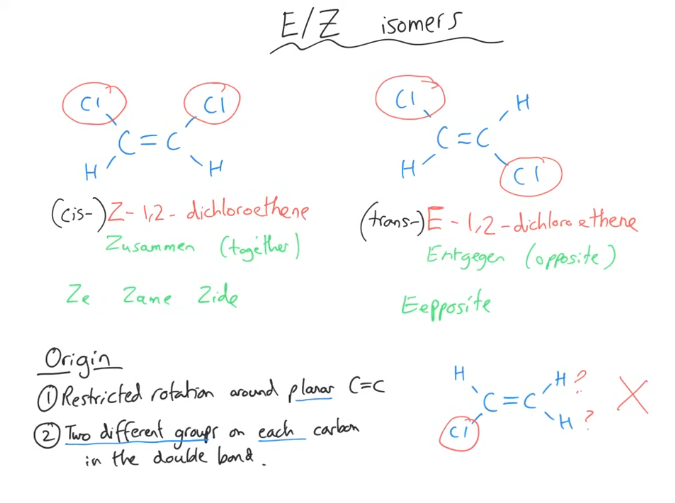
Tell me about the 1st type of stereoisomer
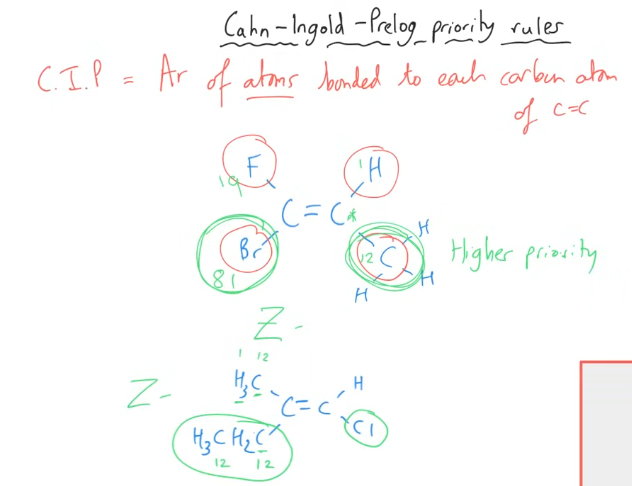
Key set of rules with Isomers
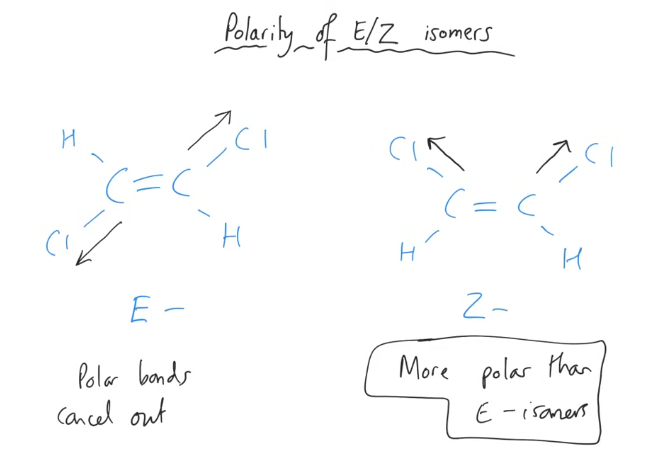
Polarity with E & Z isomers
What are stereoisomerism? (Definition, not the types)
Same molecular and structural formula, but different arrangement.
Application:
Concerns alkenes (C=C), where the same group is not attached to the same carbon
Does not concern straight alkanes, as they can rotate freely
But it does concern cycloalkane, because the ring prevents rotation, rather than a C=C
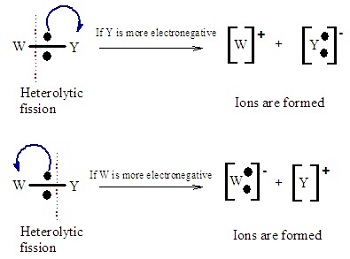
The two types of bond cleavage
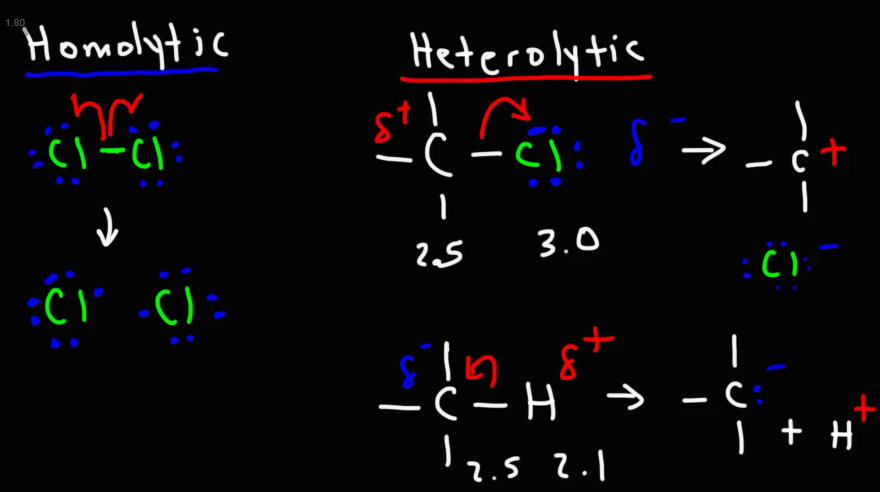
What are nucleophiles?
Electron pair donors
They contain either a lone electron pair or a negative charge, these are freed during reactions.
Examples include halide ions, oxygen, and nitrogen
What are electrophiles?
Electron pair acceptors.
They are electron deficient, and accept electrons during reactions.
Examples include H+, Alkenes, and benzene rings
What are radicals?
Species with 1+ unpaired electrons.
This makes them highly reactive, they do not donate or accept electron pairs, but can react with most chemical bonds, such as unpolarised C-H, or C-C bonds.
Examples include Cl atom
Classifications for organic reactions

“A HORSE” is an acronym for this
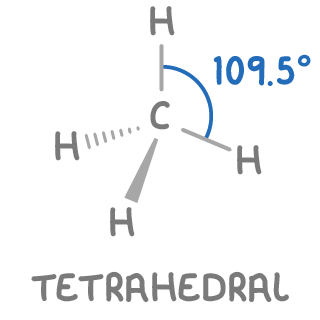
What is the standard geometry for carbon?
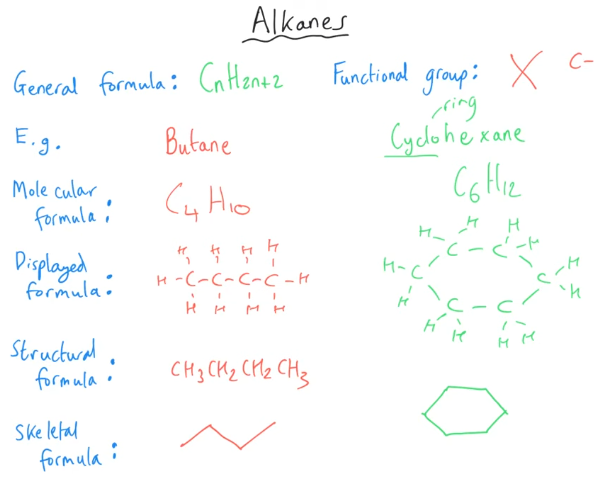
What are the key features of alkanes?
Key features of alkanes include:
They consist solely of carbon and hydrogen atoms (thus called hydrocarbons).
Each carbon atom forms four single bonds, known as sigma bonds (σ-bonds).
σ-bonds are formed by the direct overlap of atomic orbitals between the bonding atoms (C–C and C–H).
Alkanes are fully "saturated" with hydrogen, meaning they contain no double or triple bonds.
The first thing that impacts boiling points with alkanes
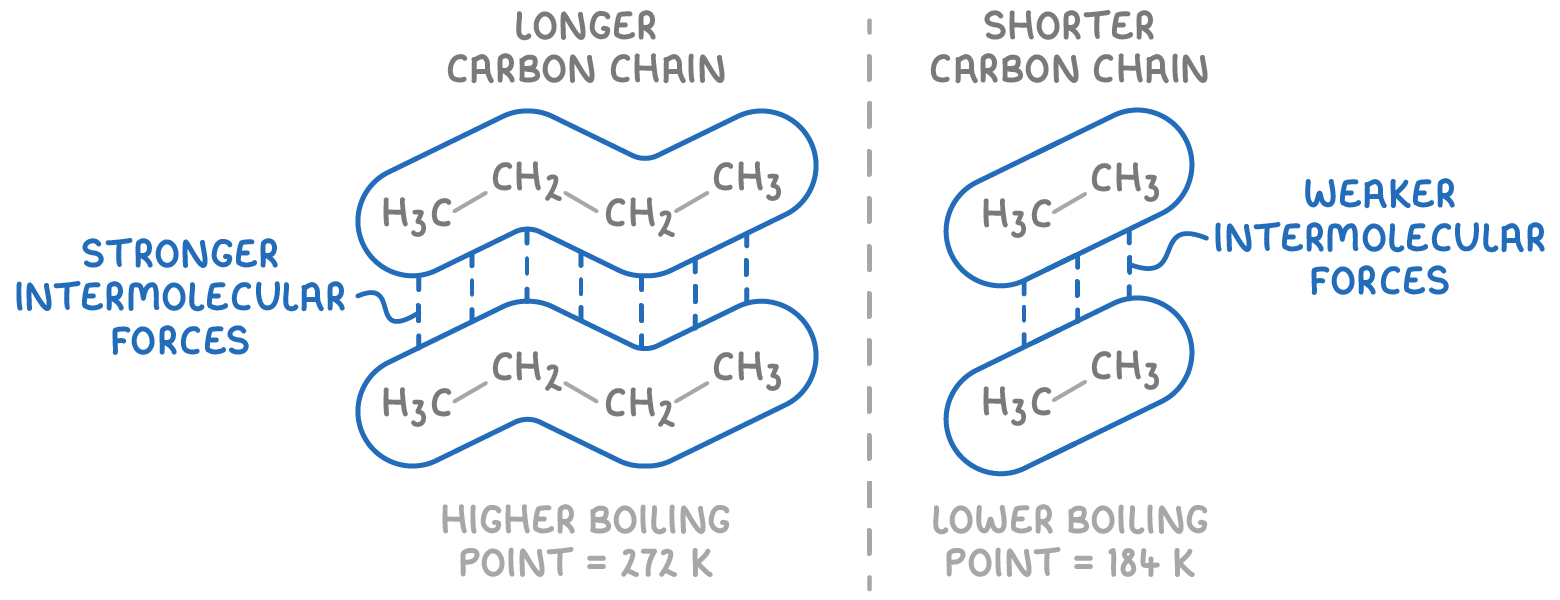

The 2nd thing that impacts boiling points with Alkanes
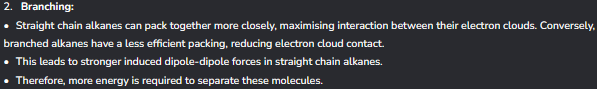
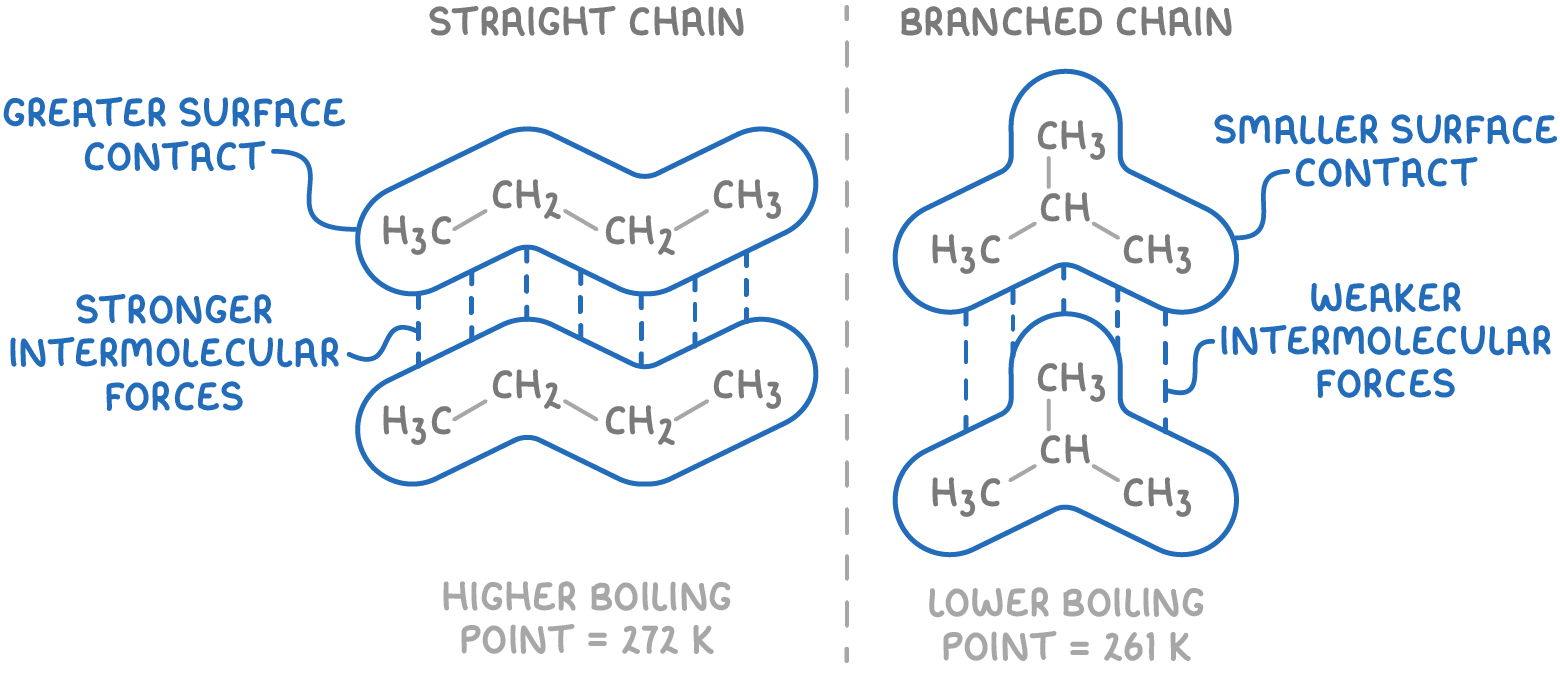

What is the general case for alkanes relating to reactivity?

Complete combustion of alkanes

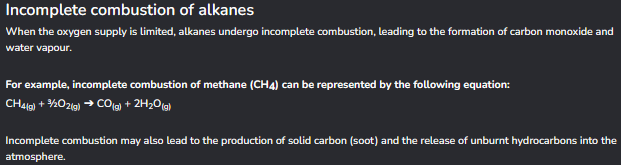
Incomplete combustion of alkanes
What is needed for the halogenation of alkanes?
Halogens react with alkanes in special light-induced reactions called photochemical reactions.
For the reaction to occur:
Ultraviolet (UV) light must be present.
This UV light provides the activation energy to start the reaction.
The overall reaction is a substitution, where a hydrogen atom in the alkane molecule is replaced by a halogen atom like chlorine or bromine.
By what mechanism does halogenation of alkanes happen?
Photochemical halogenation of alkanes follows a three-step free radical substitution mechanism:
Initiation - UV light produces reactive radicals.
Propagation - Radicals react in a chain reaction.
Termination - Radicals join to form stable molecules.
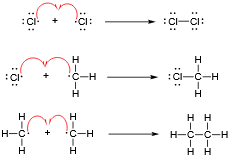
Tell me about stage 1 of the Halogenation of Methane
Stage 1 - Initiation
UV light breaks the Cl-Cl bond in chlorine via homolytic fission to give two chlorine radicals (Cl•):
Cl2 ➔ 2Cl•The unpaired electron makes Cl• highly reactive.
Tell me about stage 2 of the Halogenation of Methane
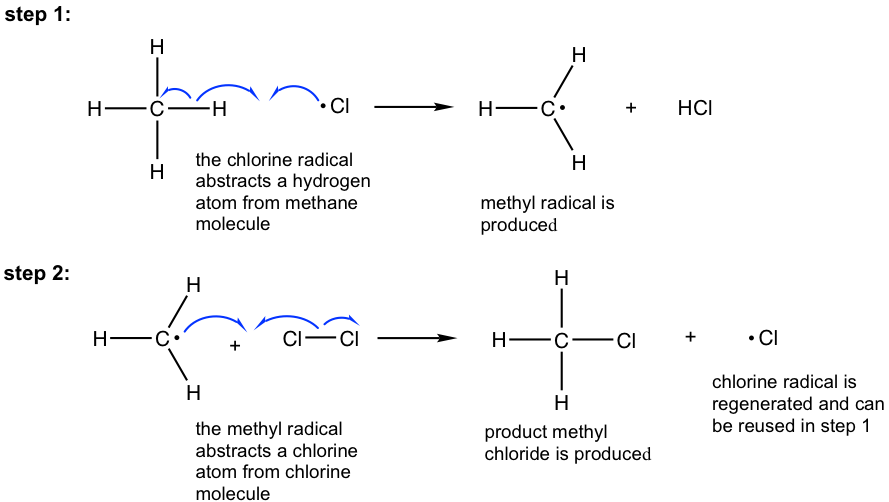
Stage 2 - Propagation
The chlorine radical attacks a methane molecule in a substitution reaction:
Cl• + CH4 ➔ CH3• + HClThe methyl radical (CH3•) attacks another chlorine molecule:
CH3• + Cl2 ➔ CH3Cl + Cl•This propagation cycle continues until reagents are used up.
Curly arrow diagram propagation with Halogenation of Methane?
Tell me about stage 3 of the Halogenation of Methane
Stage 3 - Termination
Two radicals join to form a stable covalent bond:
Cl• + CH3• ➔ CH3ClOther combinations like 2CH3• ➔ C2H6 or 2Cl• ➔ Cl2 are also possible.
Curly arrow diagram, termination halogenation of methane.
Applying Markovnikov’s rule quickly
With a hydrogen halide:
The hydrogen will attach to the carbon which has the most hydrogens.
The halide will go to the other carbon involved in the carbon bond.
What are the two main possible outcomes for the Halogenation of Methane?

Problems with producing haloalkanes from Alkanes?
What is done to combat this?
1. Production of product mixtures
With an excess of halogen, additional substitution reactions can occur.
For example, chloromethane (CH3Cl) can undergo further substitution:
Cl• + CH3Cl ➔ CH2Cl• + HCl
CH2Cl• + Cl2 ➔ CH2Cl2 + Cl•
This results in a mixture of halogenoalkanes (e.g., CH3Cl, CH2Cl2, CHCl3, CCl4) that must be separated.
2. Formation of multiple isomers
The propagating radical can substitute at any position along a carbon chain.
This leads to the production of various positional isomers.
For example, halogenation of propane yields a mixture of 1-chloropropane and 2-chloropropane.
To maximise the yield of a desired halogenoalkane, it's advisable to use a significant excess of the alkane relative to the halogen, minimising further substitution reactions.
3 Similarities between alkanes and alkenes
Melting and Boiling increase as you go down the group
They are both hydrocarbons (only contain carbon & hydrogen)
They are both insoluble in water
Key features of Alkenes
Alkenes are a class of hydrocarbons containing carbon-carbon double bonds. Their general formula is CnH2n, where n represents the number of carbon atoms.
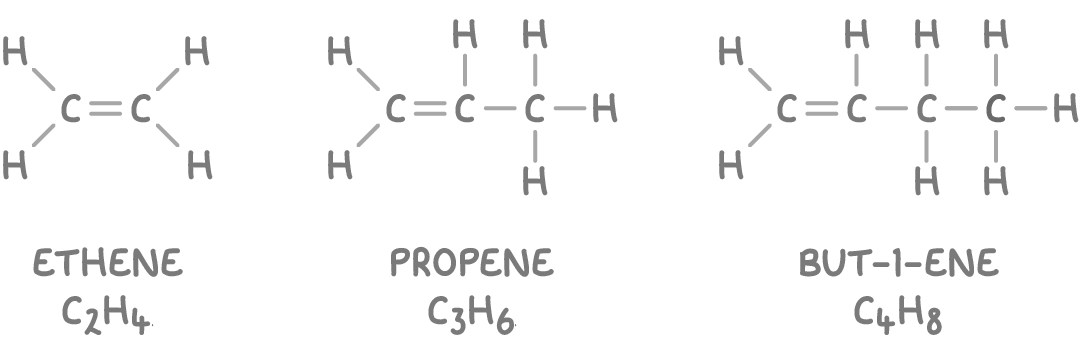
They consist of carbon and hydrogen atoms (hydrocarbons).
Each alkene molecule contains at least one carbon-carbon double bond.
The double bond makes them "unsaturated", enabling them to participate in addition reactions.
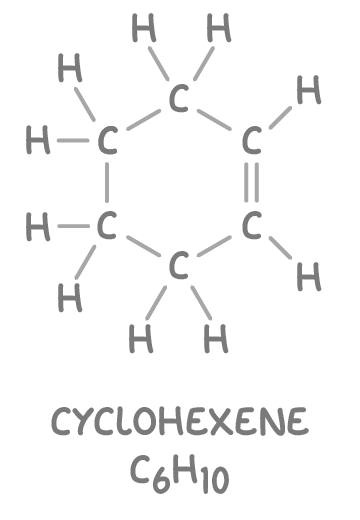
Note that cyclic alkenes such as cyclohexene (C6H10) have two fewer hydrogen atoms compared to acyclic alkenes with an equivalent number of carbon atoms. The general formula of cyclic alkenes is CnH2n-2.
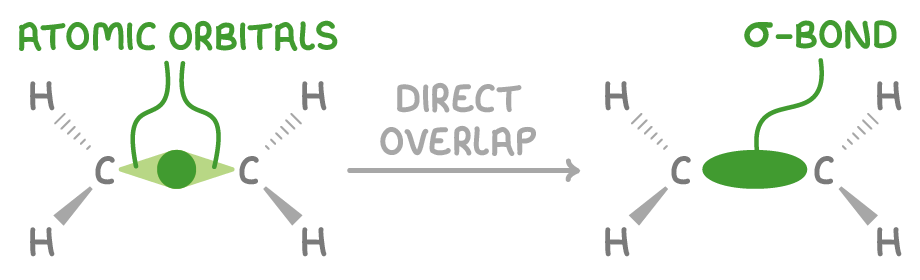
What is the stronger bonds between carbons?
A sigma (σ) bond:
This is a strong bond formed by the head-on overlap of carbon s orbitals.
It has a high electron density situated directly between the nuclei.
It is very strong, possessing the highest bond enthalpy among single bonds.
What is the weaker bond that can form between carbons?
A pi (π) bond:
This bond is created by the sideways overlap of adjacent p orbitals.
Its electron density is distributed above and below the bond axis.
It has a lower electron density and bond enthalpy compared to the sigma bond.
The presence of the pi bond restricts rotation around the C=C bond, as rotation would disrupt the parallel overlap of the p orbitals, leading to a significant increase in energy.
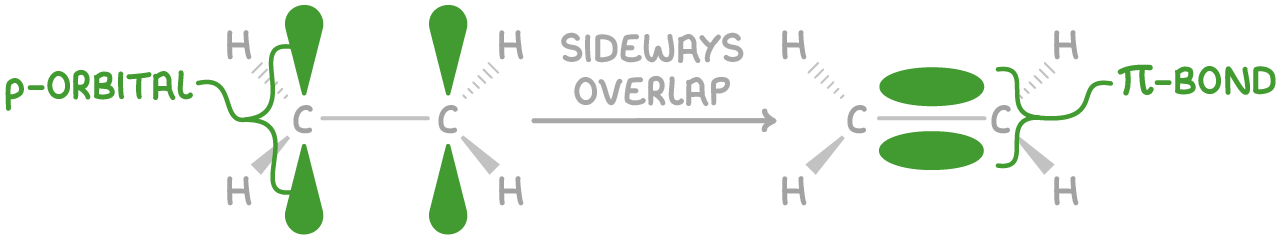
How is the bonding carbon to carbon different in Alkanes, Alkenes and Alkynes?
Alkanes: 1 sigma bond
Alkenes: 1 sigma bond, and 1 pi bond
Alkynes: 1 sigma bond, and 2 pi bonds
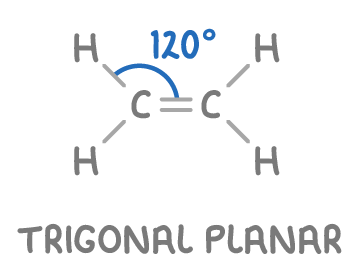
Bond geometry around an alkene’s double bond?
In alkenes, each carbon atom within the C=C double bond has:
Three regions of electron density.
These regions are arranged in a trigonal planar shape due to equal repulsion between them.
The bond angles around the carbon atoms are approximately 120°.
Compare the reactivity of Alkenes vs Alkanes
Alkanes contain only strong sigma (σ) bonds between carbon atoms.
These nonpolar sigma bonds contribute to low reactivity in alkanes:
Their high bond enthalpy makes alkane bonds difficult break.
They do not attract reactive nucleophiles and electrophiles.
By contrast, alkenes contain a pi (π) bond in their C=C double bond.
Three key features make this pi bond more reactive:
High electron density - Makes pi electrons highly accessible for reactions.
Protruding shape - Allows electrophiles to readily attack pi electrons.
Lower bond enthalpy - Requires less energy to break than sigma bonds.
These structural features of the pi bond give alkenes vastly greater reactivity over alkanes. The pi bond enables alkenes to readily undergo addition reactions across the C=C double bond.
How can 2 of the “same” alkenes be different from each other.
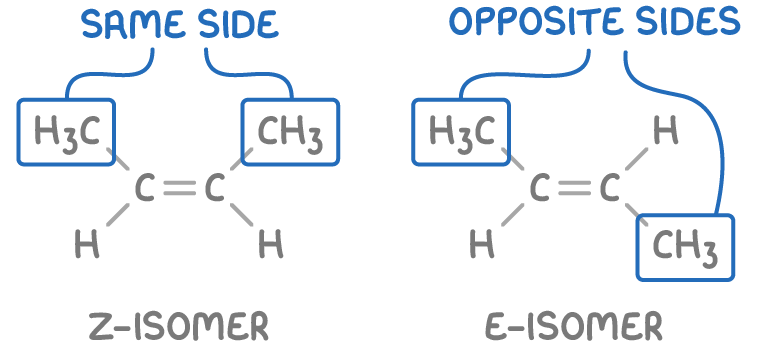
Z is for “zusammen”, meaning “together” in German, so the groups of higher interest are on the same side of the double bond.
“Ze Zame Zide”
E is for “entgegen”, meaning “opposite” in German, so the groups of higher interest are on different sides of the double bond.
“Eopposite”
How do the rules for variants of the “same” alkene actually work?
Step 1 - Priority ranking
Assign priority to the groups attached to each of the double-bonded carbons based on the atomic number. The group with the highest atomic number receives the highest priority.
Step 2 - Assign E-Z configuration
With priorities set, label the stereoisomer with the highest-ranking groups across the C=C bond as the E-isomer. The stereoisomer with the highest-ranking groups on the same side of the bond is the Z-isomer.
Special cases:
If the attached atoms are of the same atomic number, the priority is determined by considering the next atom in the chain.
If you have the same group on the same side of the molecule, there are no E/Z isomers, there is just one.
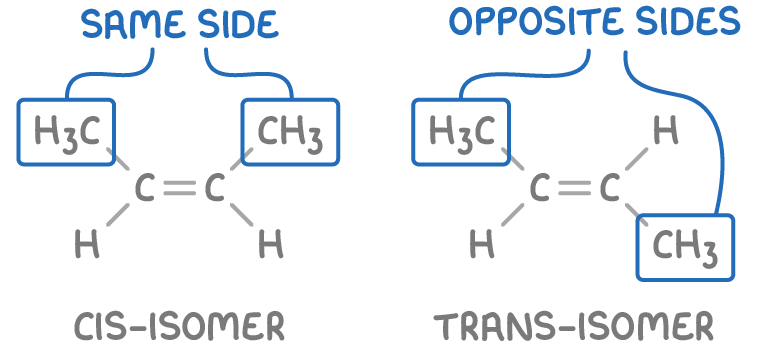
Why is there a second system for variants of the “same” alkene?
This is only used when at least there is a pair of the same functional groups (one on each carbon atom). If this is true, you use the Cis and Trans grouping system
Cis - Identical groups are on the same side.
Trans - Identical groups are on opposite sides.
How do you convert an alkene to an alkane?
Nickel Catalyst at 150°C, in the presence of H2 will cause the double bond to break, and an alkane to be formed.

Points about all the addition reaction with Alkenes?
Electrophilic Addition Reaction
Alkenes undergo a type of reaction called electrophilic addition across their carbon-carbon double bond. In this process:
The π bond of the C=C double bond breaks.
Atoms or groups add across the carbon atoms.
This happens because the C=C double bond is rich in electrons, making it a target for electrophiles - electron pair acceptors that are attracted to areas with a high electron density.
Common electrophiles that add to alkenes include:
Polar molecules, where the positive δ+ atom is attracted to the C=C electrons, such as the Hδ+ of H-Br.
Halogens such as chlorine and bromine.
Describe how an alkene will react with a Halogen.
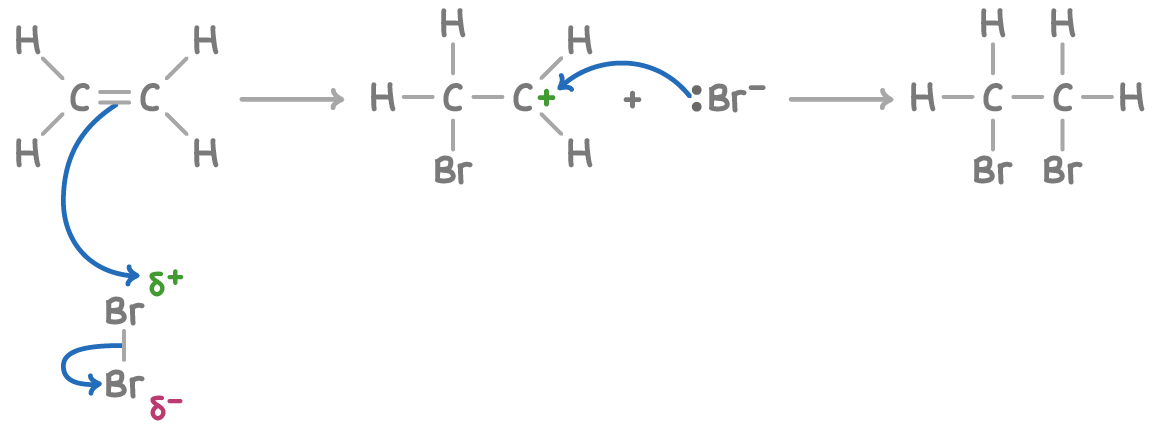
High electron density in C=C bond → electrons in bromine are repelled → bromine is polarised.
Heterolytic fission of Br-Br→ 1 Br takes the bonding pair → Br+ & Br- form.
Br+ bonds the carbon → forming carbocation intermediate
Br- bonds to the carbocation → 1,2 dibromethane is produced
How to test for alkenes
Bromine water tests for carbon-carbon double bonds.
The reaction of bromine with alkenes is a useful test for C=C bonds.
Bromine water, an orange solution of bromine, reacts with an alkene:
The alkene reacts with bromine via electrophilic addition.
This reaction removes the orange colour as the bromine is consumed.
The product is a colourless dibromoalkane solution.
The rapid decolourisation of orange bromine water indicates the presence of carbon-carbon double bonds, offering a simple test for alkenes.
Process to from an alcohol from an alkene?
An example?
The reality?
Hydration to form alcohols
Under the influence of a phosphoric(V) acid catalyst, alkenes react with steam at 300°C and 60-70 atm pressure, undergoing hydration to form alcohols.
For example, ethene is hydrated to produce ethanol:
H2C=CH2(g) + H2O(g) ⇌ CH3CH2OH(g)
This reaction is significant in the industrial production of ethanol from ethene.
A limitation is the reaction's reversible nature, with only about 5% yield. However, recycling unreacted ethene can increase overall yields to over 95%.
Addition of hydrogen Halides
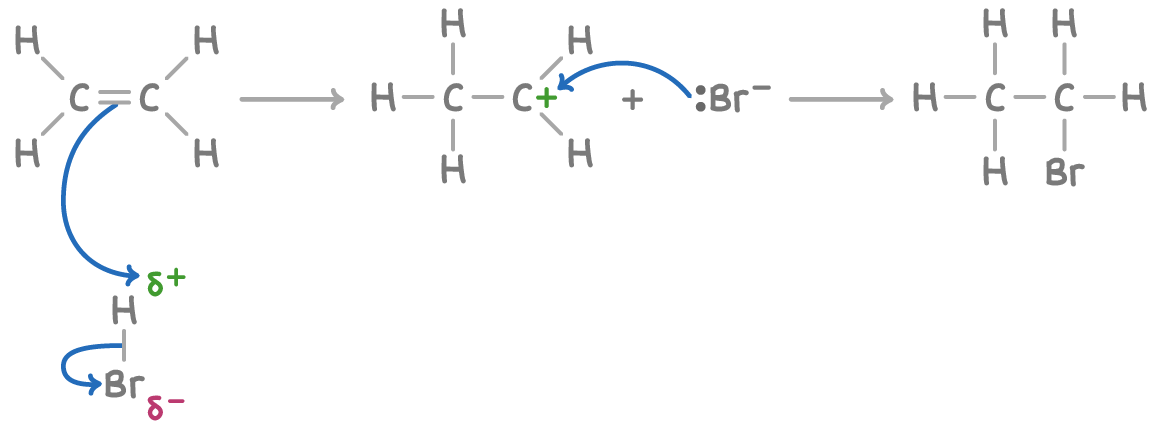
High density of electrons C=C → H+ in polar HBr is attracted
Heterolytic fission → H+ bonds to a C → Carbocation intermediate is formed
Br- → is attracted to Carbocation → its bonds → bromoethane is produced
Types of carbo-cations?
There are 3 types of Carbocations:
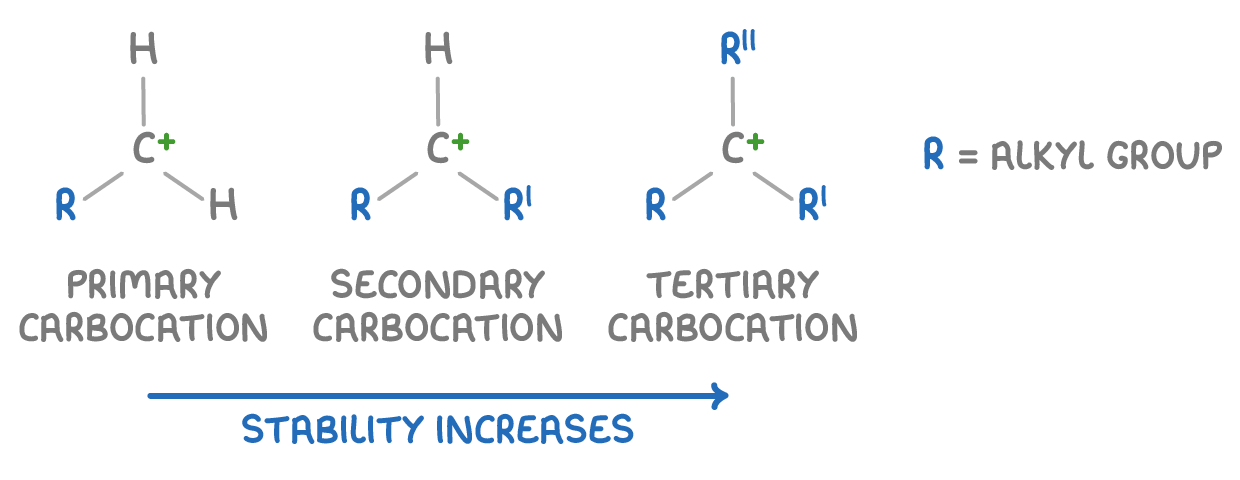
Alkyl groups stabilise the positive charge through induction effects, with more groups giving more stability.
How to predict what will be the major product in a hydrogen halide addition?
Who’s rule was this?
An example?
The major haloalkane product will form via the most stable carbocation intermediate.
Markownikoff's rule:
"The major product has hydrogen adding to the carbon with the most hydrogens already attached."
For example, in the reaction of propene with HBr, the secondary carbocation forms preferentially, making 2-bromopropane the major product.
How do Alkenes polymerise?
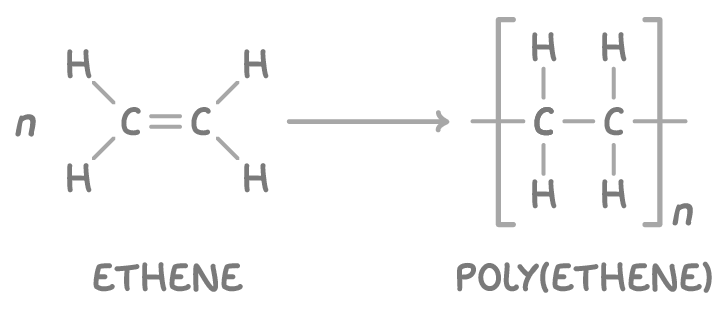
This bond can open up, allowing alkene molecules to join end-to-end and form long chains called polymers.
The individual alkene molecules are called monomers.
Joining many monomers together is called polymerisation.
Polymers made this way are called addition polymers because the double bond opens up, and monomer units are added to the chain.
Curly arrow diagram with the 2 types of fission

How to draw polymer’s repeating units
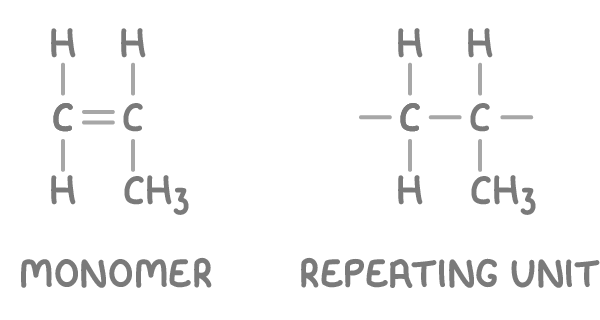
Polymers are made up of smaller repeating units. A repeating unit is the smallest section of a polymer chain that repeats over and over.
To draw the repeating unit from given monomer:
Replace the carbon-carbon double bond with a single bond.
Extend single bonds from each carbon atom to represent attachment sites within the polymer chain.
How to determine monomer from polymer section?
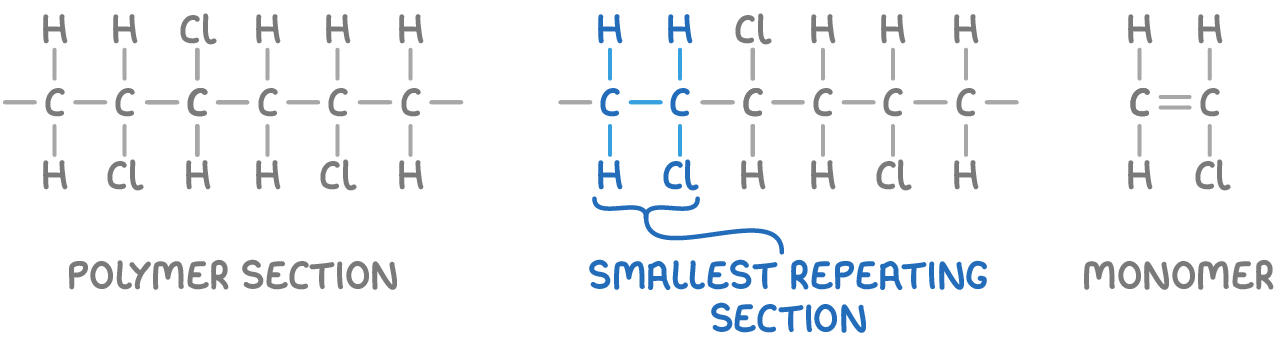
To deduce the monomer from a given polymer section:
Identify the smallest section that repeats in the full polymer chain.
Replace the carbon-carbon single bond with a double bond.
How to name addition polymers?
Addition polymers made from alkenes follow systematic naming rules:
Take the name of the alkene monomer.
Enclose the monomer name in brackets.
Add the prefix "poly".
For example, the addition polymer made from butene is poly(butene).
Addition polymers made from alkenes are called polyalkenes.
1st normal way of disposing of plastics?
Incineration

Incinerating waste plastics in specialised facilities can generate useful heat or electricity.
Advantages of first normal plastic disposal method?
Incineration
Advantages |
|---|
1. Recovers energy from waste plastics, reducing reliance on fossil fuels |
2. Substantially reduces the volume of plastic waste, minimising landfill requirements |
Disadvantage of first normal plastic disposal method?
Incineration
Disadvantages |
|---|
1. Generates toxic waste products, such as HCl, which require costly removal processes to prevent environmental damage |
2. Releases greenhouse gases, primarily CO2, contributing to climate change |
3. Incineration is an energy intensive process, partly offsetting the energy recovered |
2nd normal way of disposing plastics?
Recycling

Compressed plastic bottles ready for recycling.
Mechanical recycling - This involves sorting, cleaning, and remolding waste plastics into new objects without significantly altering the chemical structure of the polymer.
Feedstock recycling - This process breaks down polymers into their constituent monomers through cracking. These monomers can then be used as raw materials for the synthesis of new plastics and other organic chemicals.
Advantage of second normal plastic disposal method?
Recycling
Advantages |
|---|
1. Conserves valuable crude oil resources by reducing the need for raw materials |
2. Typically, results in lower CO2 emissions compared to incineration and production from raw materials |
3. Can be more economical than producing new polymers, especially when oil prices are high |
4. Reduces the amount of waste sent to landfills |
Disadvantage of second normal plastic disposal method?
Recycling
Disadvantages |
|---|
1. Requires complex and costly multistep processes for sorting, cleaning, and reprocessing waste plastics |
2. Contamination from additives, dyes, or other polymers can compromise the quality and performance of recycled products |
3. Recycled plastics may have limited applications due to reduced mechanical properties and potential contamination issues |
What was created to combat the impact of plastics?
To address the challenges posed by non-biodegradable polymers, researchers have developed degradable polymers that decompose more quickly, offering environmental benefits by reducing the accumulation of persistent plastic waste.
1st type of eco-friendlier plastic?
Biodegradable polymers:
Incorporate starch granules or are derived from plant-based monomers, making them more susceptible to digestion by microorganisms.
Polyamides and polyesters can decompose through acid hydrolysis under the hot, acidic conditions often found in landfills.
2nd type of eco friendlier plastic?
Photodegradable polymers:
Contain light-sensitive carbonyl groups that absorb UV light, weakening nearby bonds and causing the polymer to fragment into smaller pieces.
Fragmentation accelerates the biodegradation process.
Have limited effectiveness in landfill environments where waste is typically buried, blocking light and preventing photodegradation.
What are London forces?
What exactly is an alcohol?
Alcohols are organic compounds that contain a hydroxyl (-OH) functional group bonded to a carbon atom.
The general formula for alcohols is:
CnH2n+1OH
Where 'n' represents the number of carbon atoms.
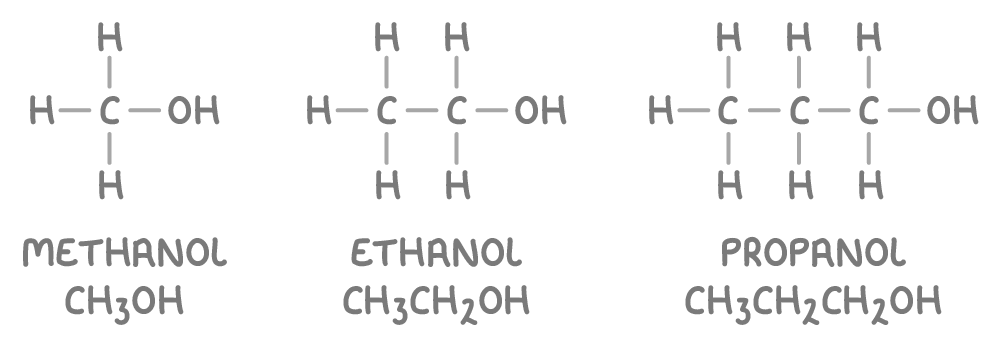
How to classify alcohols?
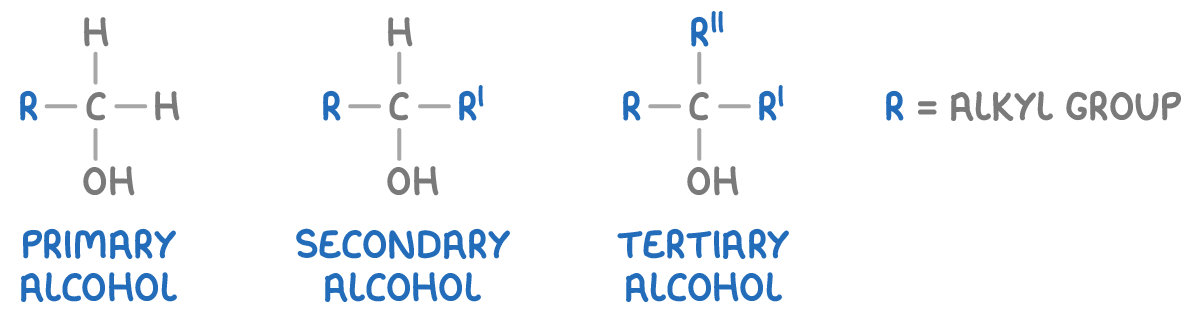
Primary alcohols - The -OH group is attached to a carbon atom that is bonded to only one alkyl group (or no alkyl groups).
Secondary alcohols - The -OH group is attached to a carbon atom that is bonded to two alkyl groups.
Tertiary alcohols - The -OH group is attached to a carbon atom that is bonded to three alkyl groups.
What special types of bonds can Alchols form?
Hydrogen Bonds
OH bond is polar, as the O is more electronegative than H
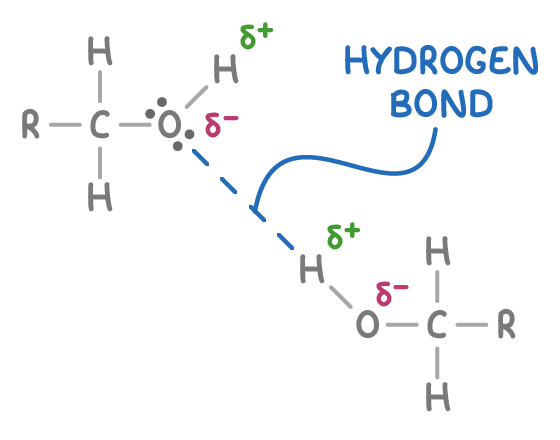
Partial positive H (δ+) can form hydrogen bonds with lone pairs on oxygen atoms in other molecules, including water molecules or other alcohol molecules (as shown above).
Explain the 1st unique property of Alcohols
Solubility
Small Alcohols are soluble, Hydroxyl forms strong hydrogen bonds with water.
Alkanes are insoluble, because they cannot do this.
Larger Alcohols have larger non-polar regions, interfering with hydroxyl-water bonds
Solubility decreases down the group
Explain the 2nd unique property of Alcohols
Volatility
The ability of alcohols to form hydrogen bonds with each other, leads to lower volatility (higher boiling points) compared to the corresponding alkene.
This is because the hydrogen bonds require more energy to break to change state from liquid to gas.
Complete combustion of Alcohols
Alcohols combust completely when burned in an excess of oxygen, breaking all C-C and C-H bonds. This results in the production of carbon dioxide and water, along with the release of heat energy.
For example, the complete combustion of ethanol is represented by the equation:
C2H5OH(l) + 3O2(g) ➔ 2CO2(g) + 3H2O(g)
Conditions for the oxidation of alcohols?
Potassium Dichromate (VI) solution (K2Cr2O7)
Acidified with dilute H2SO4
Colour change orange → green
As the reduction of Cr2O72- → Cr3+
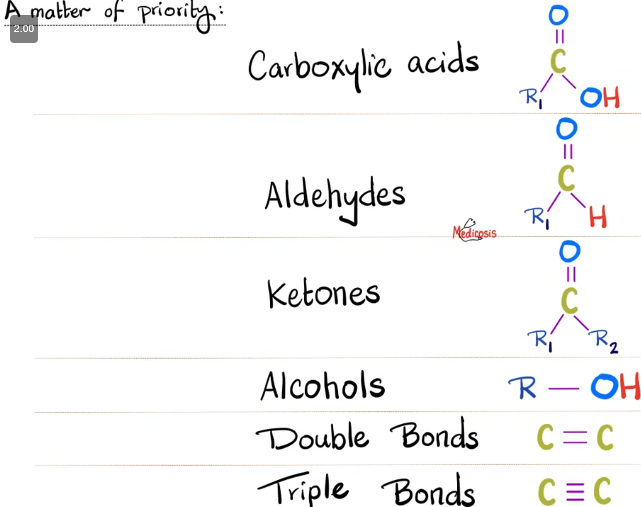
Order of IUPAC Priorities
Mapping (highest → lowest priority):
Crazy = Carboxylic acid
Sailors = Sulfonic acid
Eat = Ester
Aged = Acyl (acid) chloride
Anchovies = Amide
Near = Nitrile
All = Aldehyde
Kings = Ketone
And = Alcohol
Amuse = Amine
Angry = Alkene
Aliens = Alkyne
Eating = Ether
Hot = Halogen
Nachos = Nitro

What happens when you oxidise different types of alcohols?
The extent of oxidation depends on the alcohol's structure:
Primary alcohols are oxidised to aldehydes and then to carboxylic acids.
Secondary alcohols are oxidised to ketones only.
Tertiary alcohols do not oxidise under these conditions.