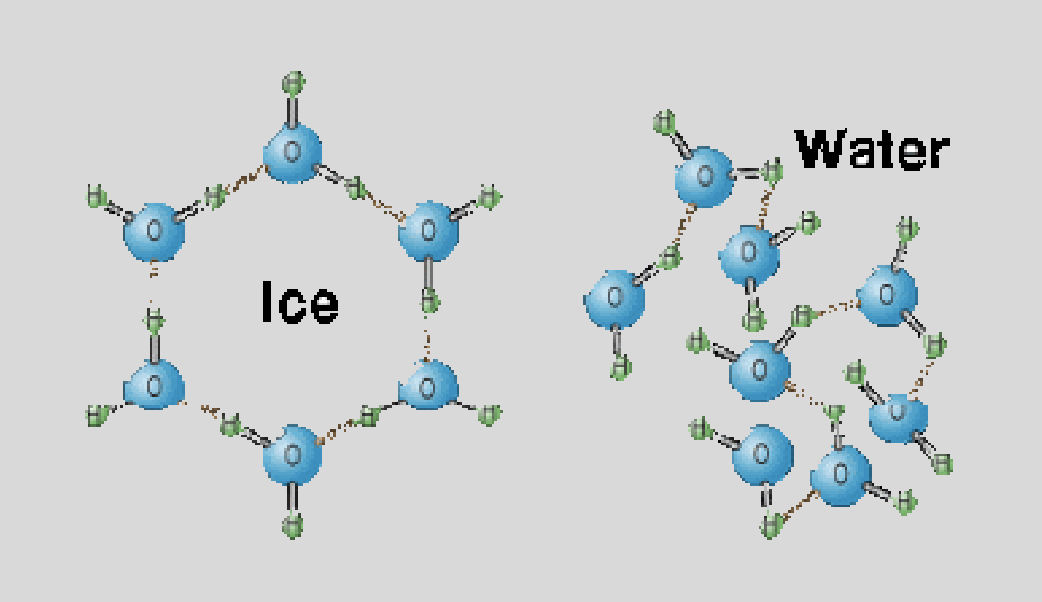
1.2 Structure of Water & Hydrogen Bonding
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
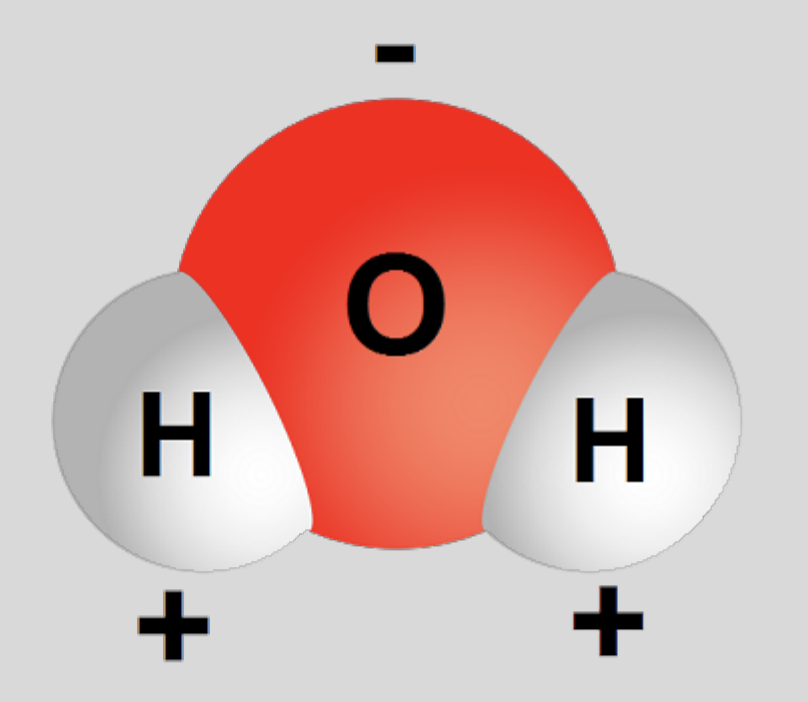
Water’s polarity
oxygen is very electronegative - “hogs electrons”
result
hydrogens have partial POSITIVE charge
oxygen has partial NEGATIVE charge
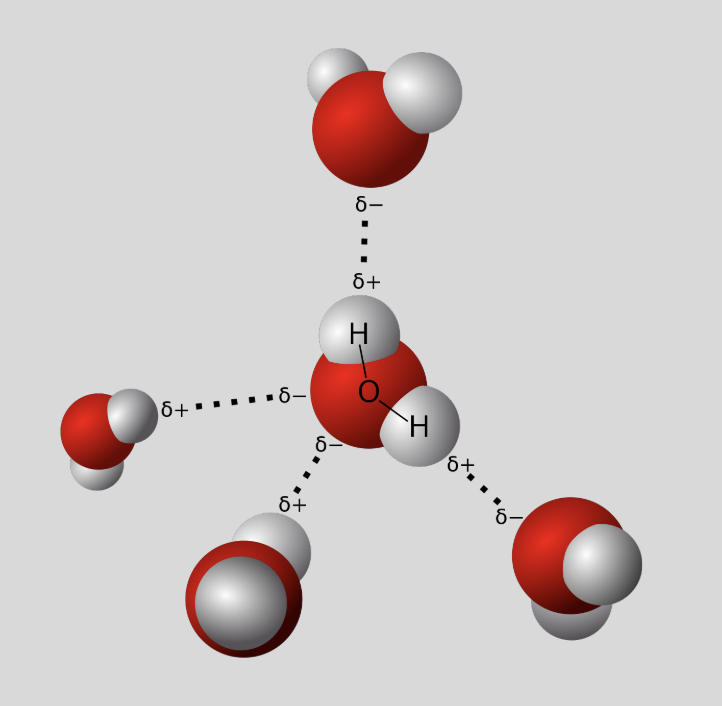
Hydrogen Bonds
weak bonds resulting from electrostatic attraction b/w molecules
direct result of polarity of water
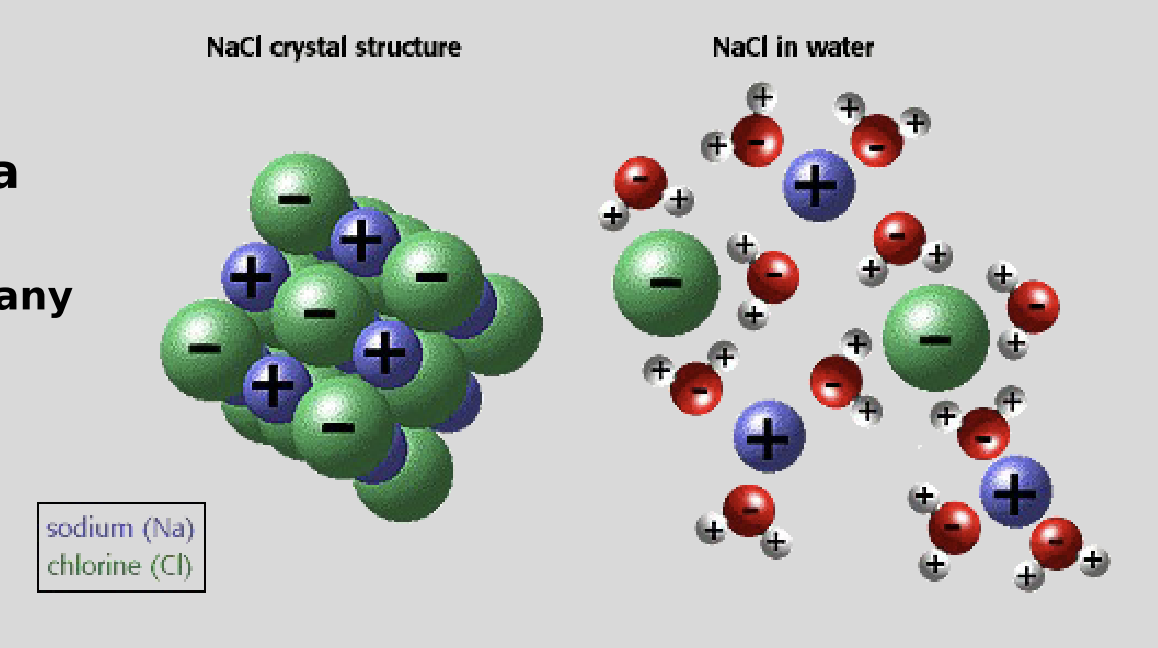
Water as a solvent
the polarity of water makes it a great solvent
“universal solvent”
dissolves many things - polar mlc., ionic mlc., NOT non-polar mlc.
ex. NaCl (ionic)
Cohesion
attraction of LIKE particles
Adhesion
attraction of DIFFERING particles
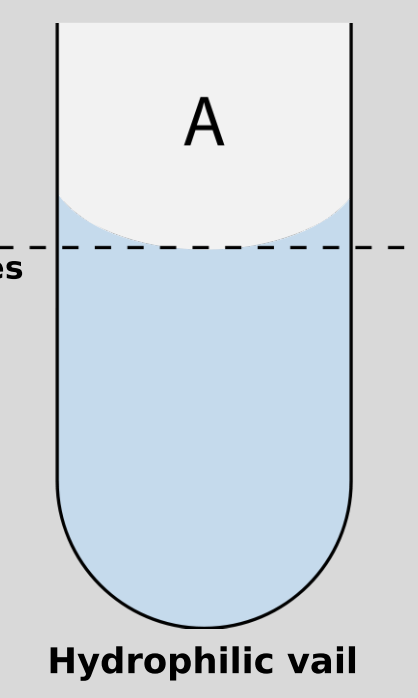
Hydrophilic
“water-loving”
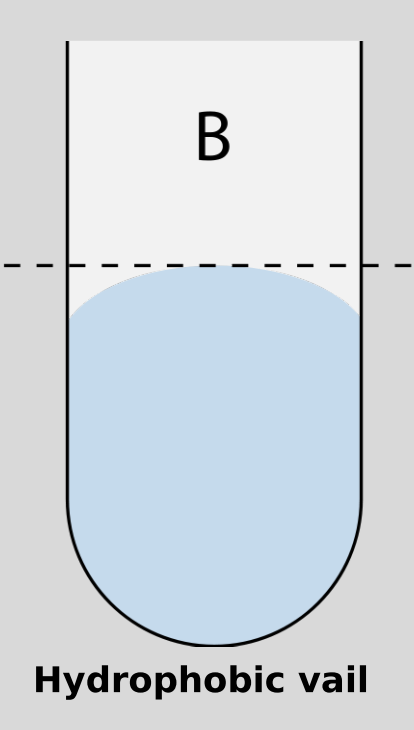
Hydrophobic
“water-fearing”
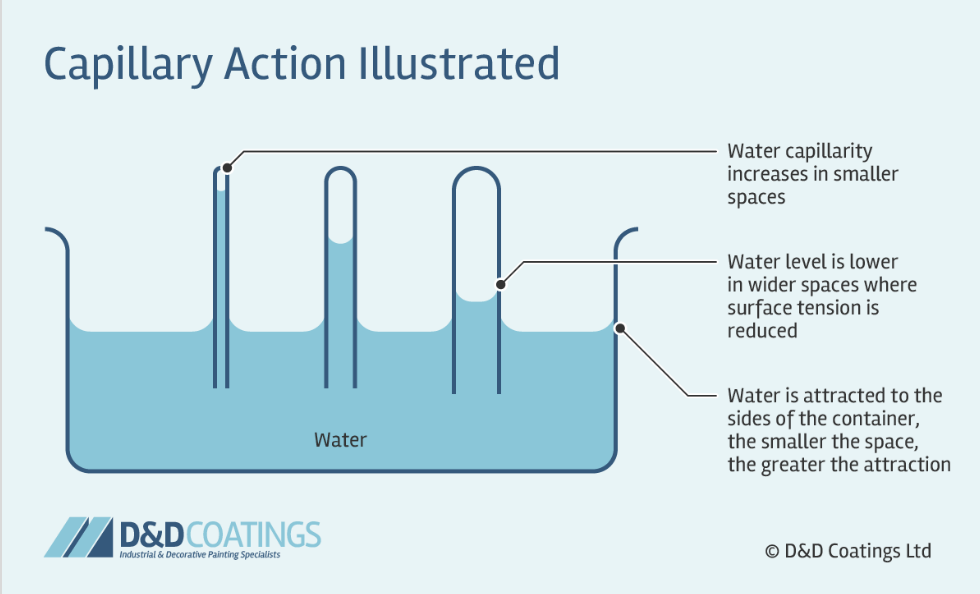
Capillary Action
act of water moving through a narrow tube against the force of gravity
Results from
cohesion (surface tension)
adhesion
Increased by
Narrowing tube’s diameter
Increasing the hydrophilicity of the tube
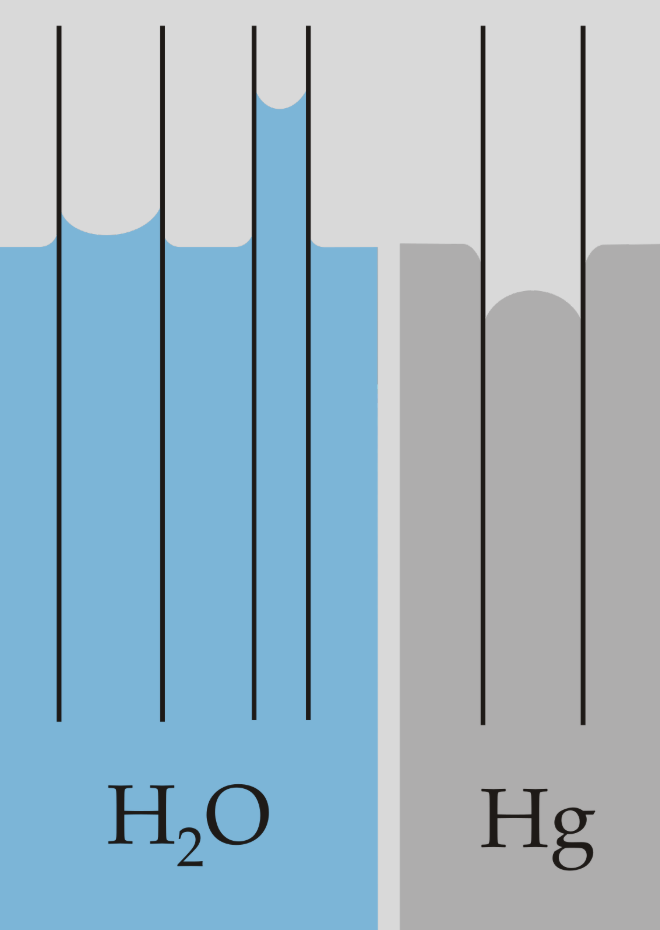
BPQ
If a tube shows capillary action in water, then in mercury the opposite effect will be observed. From this what can we infer about the properties of mercury?
The tube is hydrophilic, therefore mercury must be hydrophobic.
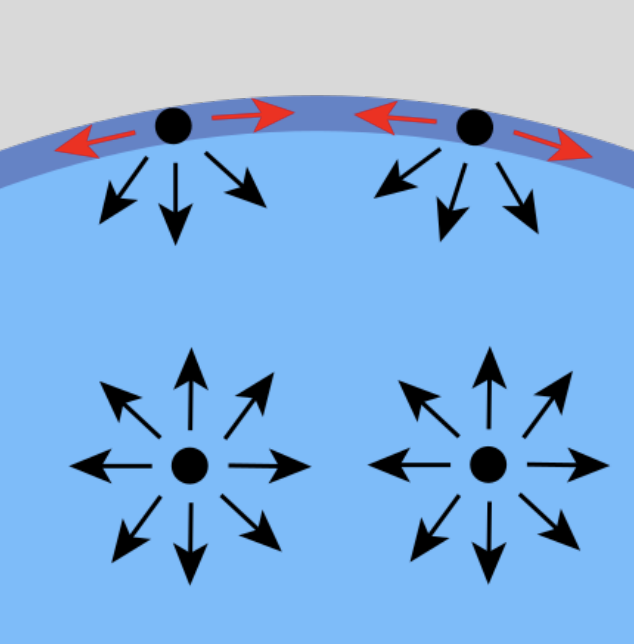
Surface Tension
enhancement of intermolecular forces on the surface
water molecules are more likely to form hydrogen bonds parallel to the surface
Heat Capacity
amount of energy needed to produce a change in temperature
water has a very high heat capacity because you need extra energy to break its hydrogen bonds
Temperature
high avg. kinetic energy = hot
low avg. kinetic energy = cold
Evaporation
process where water transitions from liquid to gas at the surface
results in a drop in temp. of the remaining liquid (evaporative cooling)
molecules with higher avg. kinetic energy evaporate
more evaporation = cooler
Evaporative Cooling
bc the average kinetic energy is higher at the surface, as it evaporates, heat is lost and the leftover fluid is cooler
Transpiration
evaporation of water from plant tissue
water replaced thru plant’s roots & stems
BPQ
What property of water allows
water to flow from the roots to
the leaves?
Capillary action allows water to flow from roots to leaves
Ice
less dense than water - floats!
water expands as it freezes