Biology Exam 1
Chapter 1
Scientific Inquiry
A Scientific Inquiry is a cycle of Exploration, Investigation, and Communication
Scientific method is a series of ordered steps which involve:
Making careful observations,
Asking critical questions,
Developing hypothesis (tentative explanations),
Making predictions,
Performing experiments
Drawing conclusions
A hypotheses makes predictions about observations that can be tested. Explanations of natural events that have been supported by many experiments are called theories.
Living organisms are complex, they are able to change in response to the environment, they are able to reproduce, and they have the capacity to evolve.
The cell theory states that all living organisms are composed of cells and that new cells can only arise from existing cells.
Both living and nonliving things share the same chemical foundations and obey the same physical laws. However the elements in the living and nonliving environments are different. (Earth’s crust has oxygen, silicon, and metals while organisms have oxygen, carbon, and hydrogen.)
Oxygen makes up more than 40% of both living organisms and the Earth’s crust.
`Evolution
A major mechanism of evolution is natural selection. It can occur when there is variation in the population, when the variation is heritable, and when some variants are more likely than others to survive, reproduce, and contribute to the next generation.
Variation can be genetic (differences in individual DNA, leading to differences in that individual’s RNA and proteins causing differences in the physical appearance.) or environmental (how the environment has affected an organism.)
Mutation is the ultimate source of genetic variation.
According to evolutionary theory, new species arise by the divergence of populations through time from a common ancestor, as a result, closely related species are likely to resemble each other more closely than they do more distantly related species. *see phylogenetic tree
human activity does affect other species.
Agriculture has increased the abundance of grapes corn, cows, and apple trees.
Our crowded environments have increased the population of rats and roaches.
We cause the extinction or decline of many animals
Ecology
Ecology is the study of how organisms interact with one another and with their environment.
Features such as anatomy, physiology, and behavior shape ecological systems.
Ex. bee chooses a flower based on its color and odor and pollinates or the trees in a rainforest all require water and nutrients and compete for the supplies of these materials. Another example is how Leafcutter ants cut slices of leaves to grow fungi that they eat because plants provide food for many types of animals.
These interactions play an important role in evolution.
Thermodynamics
The first law of thermodynamics states that energy can’t be created or destroyed but can be transformed.
All organisms get energy from the sun or chemical compounds which they transform into heat and work (moving, reproducing, and building cellular components)
The second law of thermodynamics states that the degree of disorder in the universe tends to increase.
Metabolism is all of the chemical reactions in a cell that are required to sustain life.
The amount of disorder in a system is the entropy of the system.
When small molecules are linked together to form larger molecules, the increase in entropy comes from heat loss.
Cells
The cell is the simplest biological thing that can exist on its own.
Cells are the simplest self-replicating entity that can exist as an independent unit of life.
Most cells cannot be seen by the naked eye but some special cells are quite large.
Skin cells are .1mm, nerve cells 1mm, ostrich egg 45cm
The 3 Key Features of cell is that they have an ability to store and transmit information, They have a plasma membrane that creates a distinct boundary separating the cell interior from the external environment., and they have the ability to harness energy from the environment to power cellular processes.
Cells share unity but also have diversity (Environmental variation, Genetic variation, Ecology (How organisms interact with each other and their physical environment.)
The 3 domains of life are Bacteria, Archaea, and Eukarya and they come from a single common ancestor
There are 2 types of cells: Prokaryotic and Eukaryotic (Central Dogma and Energy)
Bacteria and archaea lack a nucleus and are prokaryotes but eukarya and archaea are closely related.
Human cells consist of mostly water, but after the removal of water, the cell’s dry mass consists of carbon, oxygen, hydrogen, nitrogen, sulfur, phosphorus, and calcium.
Membrane
The cell membrane is what separates the cell from its environment
Some cells have internal membranes that divide the cell into compartments
The cell membrane surrounds all cells, controlling communication between the cell and its environment.
Eukaryotic cells also have membrane-bound organelles that form separate compartments within the cell.
Nucleic Acids/DNA and RNA
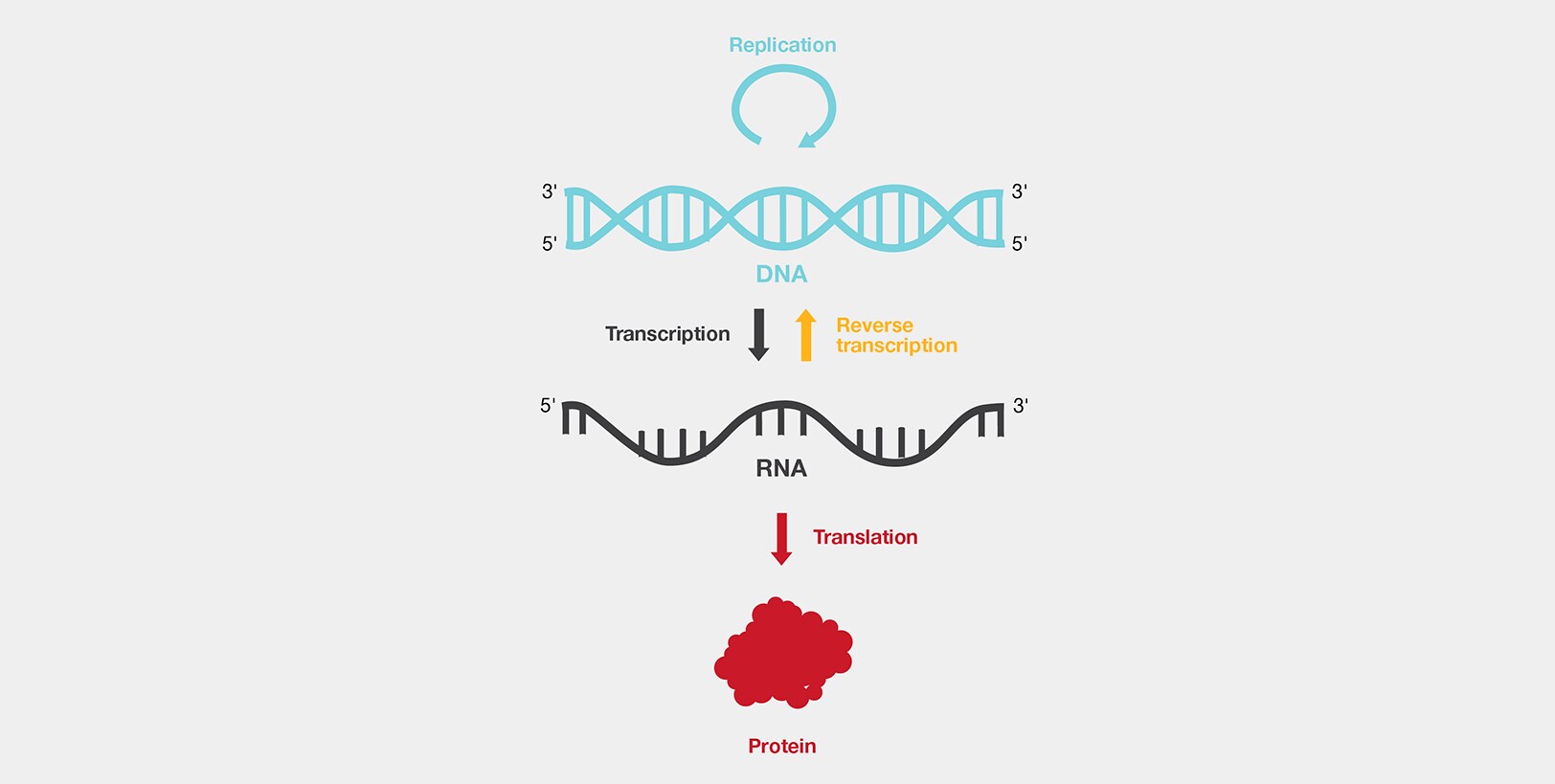
Cels contain a stable blueprint of information in a molecular form; DNA
It is a double-stranded helix, of which each strand has difference sequences of 4 different kinds of molecules which is made of a cells information.
DNA stores and helps transmit information needed by the cell.
This information can also be replicated and passed from cell to cell or fromm one organism to its progeny.
DNA is transcribed into RNA, which is then translated into protein.
A gene is the DNA sequence that corresponds to a specific protein product.
Chapter 2
Atoms and Elements
Atoms consist of protons, neutrons and electrons.
The atom’s nucleus is formed by protons (positively charged) and neutrons (negatively charged) while the electrons move around the nucleus in orbitals
Elements contain only one type of atom.
Isotopes are atoms of the same element that have different numbers of neutrons but the same proton number.
Ex. Carbon has 3 isotopes: One has 6 neutrons and it is in nature 99%, one has 7 neutrons and it occurs 1% of the time, and there is a small fraction of carbon with 8 neutrons.
There are 118 known elements: 94 are naturally occurring while 24 are human made.
The atomic number is specified by the number of protons. ex. an atom with six protons is always carbon
The number of protons plus neutrons is its atomic mass.
Elements in the same row of the periodic table have the same number of shells and, therefore, have the same number of types or orbitals.
Across a row, electrons fill the outermost shell until a full comlement of eight electrons is reached.
Vertical columns are called groups.
Members of a group have the same number of electrons in their outermost shell.
The arrangement of atoms is important, and is affected by the presence of single and double bonds.
Two molecules with the same chemical formula may be arranged differently to produce different structures and, in turn, molecules with different functions. These molecules are called isomers.
Electrons
If an atom gains or loses an electron, then it can carry a charge.
If an atom has lost an electron it is positively charged
If an atom has gained an electron, it is negatively charged.
Electrons move around the nucleus within orbitals-defined regions of space where an electron is found most of the time.
The max # of electrons is two. Atoms with more than two electrons have multiple orbitals, differing in size, shape, and distance from the nucleus.
The shells after the first one has 4 orbitals and can contain up to 8 electrons (an octet) while the first shell has one orbital and contains only 2 electrons.
Ex. Carbon 6 electrons: Two electrons occupy the first orbital in the first shell. The remain four electrons are distributed amount the four possible orbitals in the second shell with no more than two electrons in each orbital.
Ex. Hydrogen 1 electrons: The electron occupies the first orbital in the first shell.
Bonds
Atoms can combine with one another to form molecules, which are held together by chemical bonds.
Molecules tend to be the most stable when their atoms share enough electrons to occupy completely the outermost energy level, or shell
Ex. One carbon atom with four valence electrons (the electrons in the outermost shell) combines with four hydrogens, each with one valence electron, to fill the outer orbital with eight electrons.
A covalent bond forms when two atoms share a pair of electrons in a molecular orbital
The sharing of electrons most generally occurs in the outermost orbitals of the atoms.
A molecule forms when two atoms share their valence electrons with each other.
Polar covalent bonds are characterized by unequal sharing of electrons.
In water the electrons are more likely to be located near the oxygen atom than the hydrogen atoms which is due to the property of electronegativity (the ability of an atom to attract electrons
Different electronegativities between atoms cause polarity.
A covalent bond between atoms that have the same, or nearly the same, electronegativity is a nonpolar covalent bond
The atoms share the electrons equally.
Ionic bonds are formed between two oppositely charged ions.
When ionic bonds between the two form, the chlorine takes an electron from sodium, and the result is a negatively charged chlorine atom and a positively charged sodium atom. The interaction between the two occurs because opposite charges attract one another.
These two atoms are attracted to each other because of their differences in charge.
Chemical reactions are the breaking and forming of chemical bonds.
The reactants are transformed into different molecules, called products.
Water
Water is a polar molecule. It is the medium of life. Water has a partial positive charge at the hydrogens and a partial negative charge at the oxygen.
Molecules are classified based on how they react with water: Hydrophilic if they are water-loving and hydrophobic if they are not.
pH ranges from 0-14 with those lower than 7 being acidic and those higher than 7 being basic. Water has a pH of 7 and so do most of our cells.
A hydrogen bond is an interaction of a hydrogen atom and an electronegative atom.
The hydrogen, with its partial positive charge, is attracted to the partial negative charge of the oxygen.
Hydrogen bonds are depicted by the dashed lines. They are weaker than covalent bonds but they help stabilize biological molecules.
These bonds also give water molecules the property of cohesion (the tendency of the molecules to stick to one another.
Water is less dense when frozen solid than when liquid. When frozen, the molecules form a ordered crystalline lattice.
Water is able to resist temperature change more than other substances because for its temperature to increase, hydrogen bonds must first break.
Carbon
A carbon atom behaves as if it has four unpaired electrons.
In methane, each of the four valence electrons of carbon shares a new molecular orbital with the electron of one of the hydrogen atoms, forming four covalent bonds. Each of these bonds can rotate freely about its axis
Due to the shape of the orbitals, carbon is in the center, forming a tetrahedron.
Carbon atoms can also link with one another to form long chains that can be branched or form a ring structure.
Adjacent carbon atoms can also share two pairs of electrons, forming a double bond between them.
The double bond is shorter than a single bond and is not free to rotate.
Double bonds can be found in chains and ring structures as well.
Organic Molecules
The four major elements are carbon oxygen, hydrogen, and nitrogen, which makes up 94% of the dry mass.
Carbon is 52% of that dry mass and is unique because it has the ability to combine with a wide variety of molecules.
Molecules that contain carbon are called organic molecules.
There are four types of organic molecules: Proteins, nucleic acids, carbohydrates, and lipids
Stanley Miller performed an experiment using gases thought to be present in early Earth. Upon analysis of the resulting substance, there were about 20 different amino acids present.
In another experiment, Leslie Orgel placed a short nucleic acid sequence into a reaction vessel and then added individual chemically modified nucleotides. This experiment showed that nucleic acids can be synthesized experimentally from nucleotide building blocks.
Proteins
Proteins provide structural support and act as catalysts to facilitate chemical reactions.
Proteins are made of amino acid subunits.
A central carbon atom is covalently linked to four groups: Carboxyl (COOH), Amino, Hydrogen, and a R group.
The R group is what distinguishes one amino acid from another.
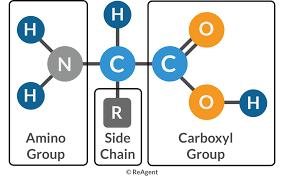
Proteins are also called polypeptides
When amino acids are linked together in a chain, they form a protein through a covalent bond called a peptide bond.
The carboxyl group releases an oxygen atom, and the nitrogen loses two hydrogen atoms to form a molecule of water when the peptide bonds are formed.
Nucleotides
Nucleotides are composed of three components: A five-carbon sugar (ribose or deoxyribose), a base containing nitrogen, one or more phosphate groups
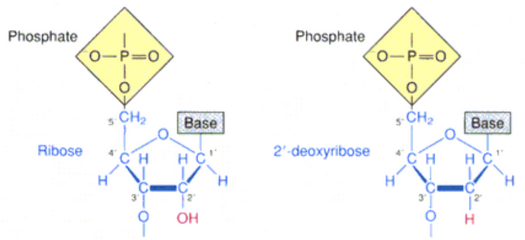
Nucleotides are the subunits of nucleic acids, like DNA and RNA
Each nucleotide contains a pyrimidine or purine base, the namesake of the nucleotide.
The bases in nucleic acids are single-ring pyrimidines (T, C, U) or double-ring purines (A, G)
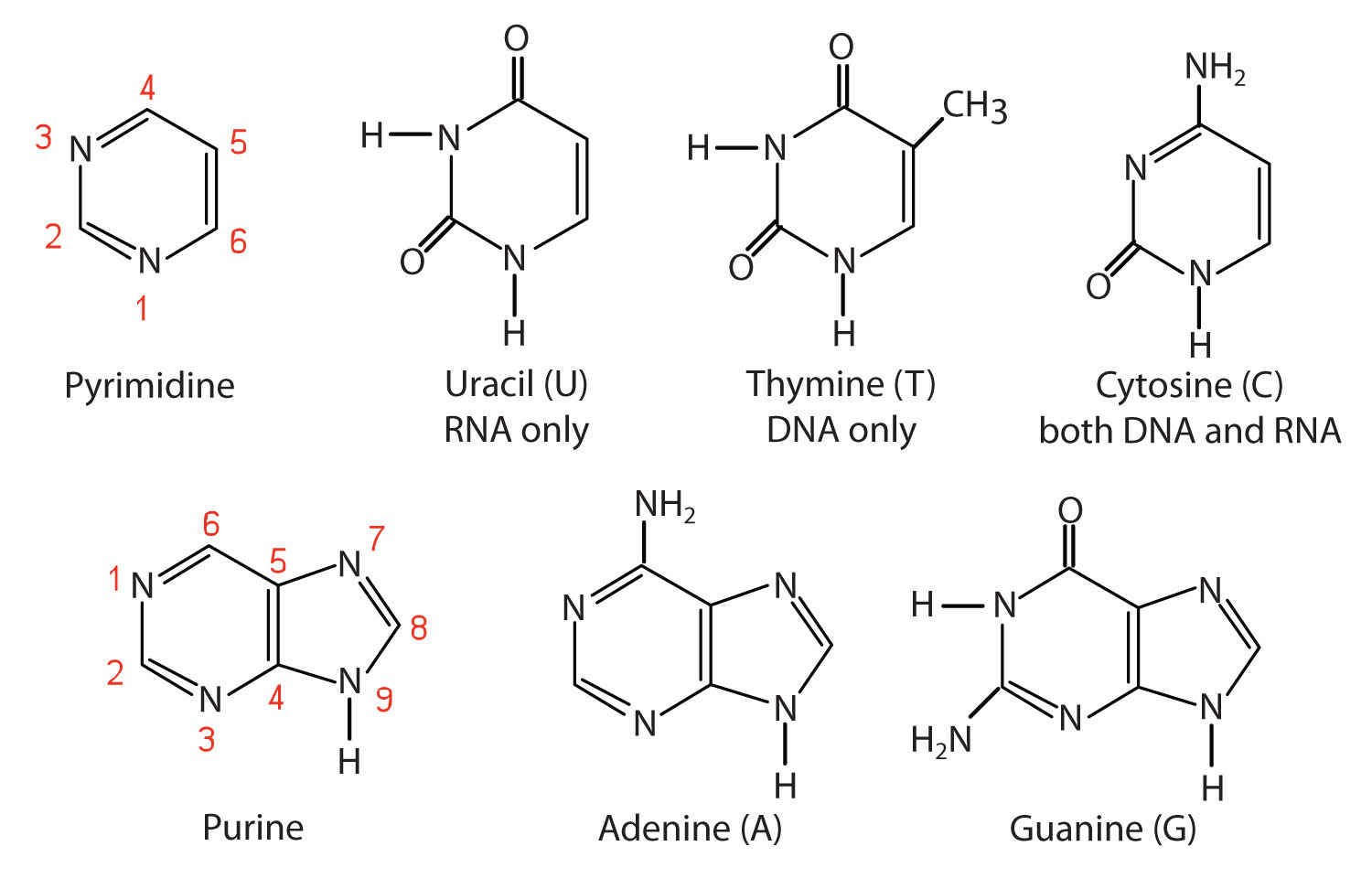
Pairs of nucleotides are joined together by phosphodiester bonds.
The phosphate group of one nucleotide is joined to the sugar unit in another nucleotide.
The formation of this bond results in the loss of a water molecule and it establishes the directionality/polarity.
DNA consists of two strands of nucleotides twisted around each other in the form of a double helix.
The bases form base-pairing A-T, G-C bonded with hydrogen bonding which makes it easier to unwind as it is weak.
Carbohydrates
Carbohydrates, or sugars, are composed of C, H, and O in a 1:2:1 ratio
Sugars containing an aldehyde group are aldose sugars, and those containing a ketone group are called ketose sugars.
A monosaccharide contains one sugar molecule.
Disaccharides contain two bonded monosaccharides.
Polysaccharides contain more than two monosacchride monomers and are formed through dehydration reactions.
The bonds that link these monosaccharides together are called glycosidic bonds.
Lipids
Lipids are defined by a property: they are all hydrophobic making them chemically diverse
Fatty acids are long chains of carbons attached to a carboxyl group at one end.
Saturated v. unsaturated fatty acids have different structure based on the presence of double bonds.
Triacylglycerols are uncharged and hydrophobic
In Van der Waals forces, the constant motion of electrons leads to regions of slight charges, and these charges are attracted to or repelled by neighboring molecules.
They are weaker than hydrogen bonds but act together to stabilize molecules and their strength is increased depending on the length of the hydrocarbon chains
The kinks in the fatty acids are caused by unsaturated carbons that have double bonds between them. which reduces tightness.
Animal fats are saturated.Without double bonds causin kinks in the structure, animal fats can stack closely together and are stabilized by more van der Waals interactions than unsaturated fats.
Steroids are composed of many carbon atoms bonded to form rings.
Cholesterol is a steroid that serves as a precursor for the synthesis of steroid hormones such as estrogen and testosterone.
Chapter 3
Cell Theory and Cell Classification
Cell Theory
All organisms are composed of cells.
The cell is the basic unit of life.
Cells arise from preexisting cells.
Prokaryotic and Eukaryotic Cells
Prokaryotic cells lack a nucleus and internal organelles.
Eukaryotic cells have a nucleus and membrane-bound organelles.
Prokaryotes | Eukaryotes | |
Nucleus | None | Yes |
Transcription location | Cytoplasm | Nucleus |
Translation location | Cytoplasm | Cytoplasm |
Cell membrane additions | Hopanoids | Sterols (cholesterol) |
Size | Small | Larger |
Ratio of surface area:volume | High | Low |
Internal organization | No organelles | Has organelles |
Cell Membrane Composition
Major type of lipid in cell membrane is phospholipids.
Phospholipids have hydrophobic, non polar regions and hydrophilic, polar regions. (having both hydrophobic and hydrophilic groups makes it amphipathic)
The head contains a glycerol, a phosphate group, and a polar group
The tail is a fatty acid tail with no charge
Lipid structures form micelles (bulky head; one hydrophobic tail) and bilayers and liposomes (small head and 2 hydrophobic tails).
Liposome
When in water, phospholipids spontaneously form enclosed bilayers called liposomes.
They can only do so in an aqueous environment with the pH approximately neutral.
Membranes are self-healing because of the spontaneous reforming
Membrane Fluidity and Components
Lipids and proteins coexist in the membrane, forming a mosaic.
Lipids move laterally within the membrane making it fluid.
Saturated vs. Unsaturated
Lipids associate with one another by Van der Waals forces between their fatty acid tails which help stabilize the membrane
This force is weak allowing the lipids to move freely and become fluid as will as the fatty acid chains being able to bend and flex (making membranes dynamic)
The strength of these interactions between tails depends on its length and the presence of double bonds.
Sat. fatty acids have no double bonds while Unsat. fatty acids do.
Cholesterol
Cholesterol is a component of animal cell membranes and is amphipathic of 4 interconnected carbon rings
Depending on the temp., cholesterol can increase or decrease membrane fluidity (ex. at normal cell temp. the interaction of the rigid structure and the fatty acid tail reduces the mobility + the fluidity of the phospholipids and membrane.)
Proteins in the Membrane
Membrane proteins serve as transporters, receptors, enzymes, and anchors.
Transporters move molecules across the membrane; receptors allow the cell to receive signals from the environment; Enzymes catalyze reactions; Anchors help maintain the shape of the cell.
Integral proteins span the membrane (transmembrane proteins) and cannot be separated from it without destroying it, while peripheral proteins are temporarily associated with either side of the membrane.
Evidence for protein fluidity includes a technique called FRAP (fluorescence recovery after photobleaching). *go to powerpoint for more info
Cell Membrane Functionality and Homeostasis
Selective Permeability
All cells are closed by a plasma membrane that bounds and defines the space of the cell
The maintenance of a constant environment is known as homeostasis
Some organisms also have a cell wall external to the plasma membrane.
Cell membranes are selectively permeable.
Transport
Diffusion is the movement of molecules from an area of high to low concentration and is the simplest movement across a membrane
Osmosis is the movement of water only from an area of high water concentration to a low water concentration across a permeable Membrane/dialysis tubing lab.
[Solute] + [Solvent] = Solution
Water can move into a cell more readily by facilitated diffusion using protein channels called aquaporins
During facilitated diffusion in cells, molecules move from high to low concentration through transporters in the membrane that are open to on or the other side of the cell.
Energy is used to move substances against their concentration gradient.
Primary active transport involves the Na+/K+ pump (anti porter) using ATP to move Na and K ions in opposite directions *more needed info in powerpoint
Secondary active transport involves a proton pump using ATP to pump protons across the cell membrane *more needed info in powerpoint
Maintaining Cell Shape and Homeostasis
Cells regulate shape and size through active transport.
A red blood cell will be affected depending on if placed in a hypertonic or hypotonic solution and the cells solve that problem by keeping their internal environment isotonic (at the same solute concentration as the outside concentration)
Contractile vacuoles in some single-celled organisms prevent cell lysis.
Cell walls provide structural support and resists the force of turgor pressure (the force exerted by water pressing on something)
In plant cells, the vacuole absorbs water and contributes to turgor pressure.
The reducement of turgor pressure and its contribution to the tissue rigidness causes plants to wilt.
*Refer to the pictures for feature of a plant and animal cell
Endomembrane System and Organelles
Endomembrane system: system of interconnected organelles
Eukaryotic cells have internal organelles surrounded by membranes.
Vesicles provide short-lived connections within the cell as they travel from one organelle to another.
With separate cell structures, the endomembrane system allows specific functions to happen
Exocytosis and endocytosis happen when vesicles merge with the cell membrane. During exocytosis, a vesicle produced within the cell can combine with the outer cell membrane to release its contents outside the cell. Conversely, endocytosis occurs when material taken into a vesicle combines with other cell organelles.
Cell Organelles
The nuclear envelope is a double membrane that surrounds the nucleus. It has 'nuclear pores' that allow communication with the rest of the cell. (connects to ER)
Endoplasmic reticulum produces lipids and proteins; network of tubules and flattened sacs
The rough ER is associated with ribosomes which are the sites of protein synthesis
The smooth ER lacks ribosomes and is the site of the formation of lipids
Organelles like the Golgi apparatus modify and sort proteins and lipids while also producing carbohydrates. it is the next stop for vesicles after the ER using flattened sacs called the cisternae
Enzymes in the Golgi modify proteins and lipids as they pass with different steps performed in a different region the golgi.
Lysosomes are vesicles from the golgi that break down unneeded macromolecules. They contain a proton pump that keeps the internal pH of the lysosome at 5 so breaking down macromolecules is easy.
Specialized Organelles
Mitochondria and chloroplasts are organelles specialized to harness energy. They contain their own genomes
Mitochondria generate ATP from chemical compounds; in almost all eukaryotic cells
Chloroplasts capture sunlight energy for photosynthesis; double membrane; inner membrane is the thylakoid; contains pigments like chlorophyll. They utilize carbon dioxide as a reactant.