CHEM 351: ACS Midterm
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
119 Terms
thermodynamics
the study of work and heat in chemistry
system
the part of the universe that’s of interest, described using macroscopic variables
macroscopic variables
pressure, volume, temperature, amount of matter
surroundings
region outside the system, sometimes where we make our measurements
universe
the system, the surroundings, and the boundary in between
extensive parameters
obtained by summing together the contributions from all the molecules in a system
N (number of particles in a system), V (total space occupied by a system), E (sum of translational, rotational, vibrational, and electronic energies)
intensive parameters
obtained by averaging the contributions from the molecules in a system
P, density, T
zeroth law of thermodynamics
if two systems are in thermal equilibrium with each other and a third system is in thermal equilibrium with one of them, then it is in thermal equilibrium with the other also
first law of thermodynamics
the total energy of an isolated system remains constant
second law of thermodynamics
when two systems are brought into thermal contact, heat flows spontaneously from the one at a higher temperature to the one at a lower temperature
third law of thermodynamics
all perfect materials have the same entropy (S) at T=0, and this value may be taken to be S = 0. At higher temperatures, S is always positive
open system
energy and matter can be exchanged between the system and its surroundings
closed system
only energy can be transferred, either as work or by heat transfer. matter cannot be exchanged
isolated system
neither energy nor matter can be exchanged
adiabatic
the system is insulated well enough, heat will not be able to get into or leave the system (work can be performed on or by the system)
q=0
diathermic
heat transfer is possible
exothermic process
a process that released energy as heat
H < 0
q < 0
endothermic process
absorbs energy as heat
H > 0
q > 0
reversible process
one which is carried out in infinitesimally small incremental steps, the system essentially remains at equilibrium at all stages during the process
irreversible process
cannot be reversed by an infinitesimal perturbation, occurs spontaneously
temperature
measures how much kinetic energy a system has, NOT a form of energy (can be used to compare energy levels → more energy = higher temperature)
heat
the energy transferred between systems caused by temperature differences
equations of state
a mathematical equation into which we can substitute two of the variables and calculate what the remaining variable must be
Boyle’s law, Charles’ law, Avogadro’s principle
limiting laws
laws that hold true within certain limits (ex: Boyle’s and Charles’ laws hold true when P approaches zero)
ideal gas law!
PV = nRT
compressibility factor, Z
at low temperatures and high pressures, gases will deviate from the ideal gas law
Z = PV/RT
the further away Z is from 1, the less ideally the gas will behave
virial equation
the equation of state for nonideal gases
Z = 1 + B/V + C/V² + …
where B, C.. are correction factors, and B has the largest correction
Boyle temperature
Z = 1, nonideal gases start to act like an ideal gas
TB = a/bR
considerations for an ideal gas
there are no repulsive or attractive forces between the gas particles
the particles are so small, their volumes are negligible
there are no interactions between particles
van der Waals equation
where a represents the pressure correction related to the magnitude of the interactions between particles, and b is the volume correction related to the size of the particle
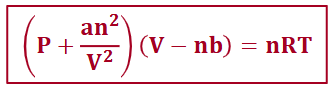
translation energy for a gas (equipartition principle)
E = 3/2kT
rotational energy for a non linear molecule (equipartition principle)
E = 3/2kT
rotational energy for a linear molecule (equipartition principle)
E = kT
vibrations of a non-linear molecule
3N - 6
vibrations of a linear molecule
3N - 5
work
done when an object moves some distance, s, due to applying a force, F; a way to transfer energy
negative work
indicates the work done results in a decrease in the system’s energy
positive work
indicates the work done results in an increase in the system’s energy
free expansion
w = 0, expansion is done against no opposing force
reversible expansion
change that can be reversed by an infinitesimal modification of a variable, let the system respond to a change before continuing

irreversible expansion
the change occurs irreversibly, suddenly changing the initial pressure to the final pressure
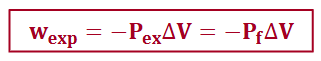
a system does maximum work when…
the process is carried out reversibly: wexp < wirr
heat capacity, C
an extensive property that includes amount of material
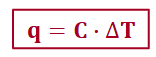
what is the difference between work and heat?
work is ordered energy transfer while heat is disordered energy transfer
internal energy
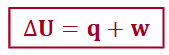
enthalpy
state function
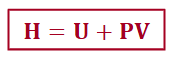
if heat capacity is independent of temperature over the range of temperatures of interest…

derivative for internal pressure
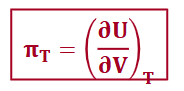
joule-thompson coefficient
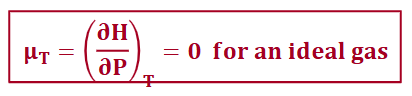
molar heat capacity at constant volume
CV = 3/2R = 12.471
molar heat capacity at constant pressure
CP = CV + R = 5/2R = 20.785
gamma for monoatomic perfect gas
5/3
gamma for linear polyatomic molecule
7/5
gamma for non-linear polyatomic molecule
4/3
phase transition
a spontaneous change of one phase into another, occurs at a specific temperature for a given pressure
hess law
standard enthalpies of individual reactions can be combined to determine the enthalpy of another reaction
kirchhoff’s law
relates the heat of a reaction at different temperatures through heat capacity
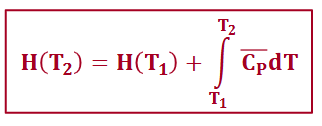
engines
have a hot source and a cold sink, some energy is lost to the cold sink as heat and not converted into work
what does heat stimulate?
random motion in the surroundings
what does work stimulate?
uniform motion of atoms in the surroundings, doesn’t change entropy
carnot cycle
reversible isothermal expansion from A to B at Thot
reversible adiabatic expansion from B to C (temperature falls from Thot to Tcold)
reversible isothermal compression from C to D at Tcold
reversible adiabatic compression from D to A (temperature rises from Tcold to Thot)
efficiency

carnot efficiency
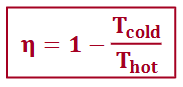
clausius inequality
more work is done when a change is reversible than irreversible (for expansion)
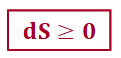
dS > 0 (isolated system)
the process is spontaneous
dS = 0 (isolated system)
the process is in equilibrium
dS < 0 (isolated system)
not allowed for a process in an isolated system
reversible change in a non-isolated system
Ssur = -Ssys, so Stot = 0
irreversible change in a non-isolated system
Ssur > -Ssys, so Stot > 0
mole fraction
ratio of the number of moles of a given gas and the total number of moles of gas
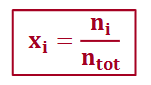
exothermic phase transitions
freezing or condensing → change in entropy of the system is negative
endothermic phase transition
melting or vaporization → change in entropy of the system is positive
trouton’s rule
empirical observation that a wide range of liquids gives approximately the same standard entropy of vaporization (about 85 J/molK)
implies that a comparable change in volume occurs when any liquid evaporates to become a gas
Boltzmann’s molecular interpretation
“an atom or a molecule can possess only certain energy values, called its ‘energy levels’.”

microstate
the ways in which the molecules of a system can be arranged while keeping the total energy constant
Helmholtz energy, A
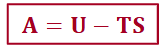
Gibbs energy, G
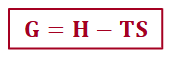
G < 0
the process is spontaneous
G = 0
the system is in equilibrium
G > 0
the process is non-spontaneous
maximum expansion work
the change in Helmholtz function (A) is equal to the maximum amount of work that a process can do at constant temperature
maximum non-expansion work
at constant temperature and pressure, the maximum non-expansion work is given by the change of the Gibbs energy (G)
from U (maxwell relationships)
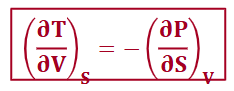
from H (maxwell relationships)

from A (maxwell relationship)
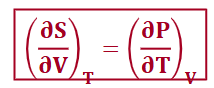
from G (maxwell relationship)
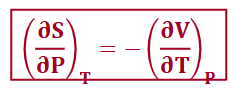
in which phase is the Gibbs energy most sensitive to changes in temperature?
gas phase
Gibbs-Helmholtz equation
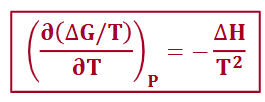
thermodynamic square mnemonic
good physicists have studied under very ambitious teachers
chemical potential
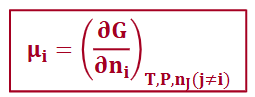
extent of reaction
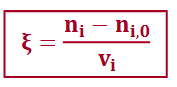
extent of reaction > 0
chemical process moves to the right (reactants)
extent of reaction < 0
chemical process moves to the left (products)

the forward reaction is spontaneous

the reverse reaction is spontaneous
G < 0
the forward reaction is spontaneous, the reaction is exergonic
G > 0
the forward reaction is non-spontaneous, the reaction is endergonic
G = 0
the reaction is in equilibrium
reaction quotient, Q
activities of products/activities of reactants
fugacity
