CHEM 266L - Understanding Checkpoints (Online + Lab Manual)
1/110
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
Which of the following can be performed outside of the fume hood? (Select all that apply.)
a) Filtering a solid
b) Running a Melting Point
c) Weighing out a solid
d) Cooling a mixture in an ice-water bath
e) Adding deionized water to your reaction mixture
a, b, and c
Which of the following behaviours protects your face and lungs? (Select all that apply.)
a) Keeping the fume hood sash as low as possible
b) Performing separatory funnel work-ups outside the fume hood when the reaction doesn't use toxic chemicals
c) Performing chemical reactions in the fume hood
d) Cooling a reaction mixture in an ice-water bath on your bench top
a and c
Which of the following scenarios will not protect your face and lungs? (Select all that apply.)
a) Cooling a reaction mixture in an ice-water bath on your bench top
b) Performing chemical reactions in the fume hood
c) Performing separatory funnel work-ups outside the fume hood when the reaction doesn't use toxic chemicals
d) Having the fume hood sash above your head
a, c, and d
Select all the reasons why the fume hood sash needs to be kept as low as possible while working in the laboratory.
a) To prevent explosions
b) To protect your forearms from chemical splashes or burns
c) To protect your face and neck from splashes or splatters that may occur
d) To prevent getting any chemicals on your legs
e) To have proper airflow and avoid the inhalation of fumes
c and e
Which of the following statements regarding protecting your skin and feet in the lab is true?
a) It is okay to have long hair loose in the lab as long as you have one free hand to hold it back
b) If it gets too hot in the lab it is okay to roll up your lab coat sleeve.
c) It is okay to wear sandles in the lab as long as your wear long pants.
d) A lab coat protects your skin and clothing from chemical burns and fires
e) Gloves only need to be worn in the lab when you are weighing out your chemicals.
d
Which of the following statements regarding protecting your skin and feet in the lab is false?
a) You should always wear a lab coat in the lab.
b) Gloves should be worn in the lab.
c) If it gets too hot in the lab it is okay to roll up your lab coat sleeve.
d) It is preferred that pants be worn in the lab to cover your legs.
e) Sandals cannot be worn in the lab.
c
Which of the following statements regarding protecting your skin and feet is false?
a) Gloves should be removed when going into the hallway.
b) Lab coat sleeves should cover your forearms.
c) Lab coats should be buttoned up.
d) It is safe to wear shorts in the lab if you have closed-toe shoes on.
e) Sandals or Crocs cannot be worn in the lab.
d
Which of the following is acceptable eye protection for the organic chemistry lab?
a) Fume hood sash
b) Eyeglasses with safety glasses on top
c) Large eyeglasses that cover your eyes
d) Sunglasses
e) Regular prescription glasses
b
Which of the following are acceptable forms of eye protection in this lab? (Select all that apply)
a) Regular prescription glasses
b) Your hands
c) Eye glasses with safety glasses on top
d) Safety glasses
e) Large eye glasses with side shields
c and d
Which of the following is not acceptable eye protection for the organic chemistry lab?
a) Eyeglasses with goggles on top
b) Regular prescription glasses
c) Regular safety glasses
d) Safety goggles
e) Eyeglasses with safety glasses on top
b
Which of the following statements regarding protecting your eyes in the organic chemistry laboratory is true?
a) If you wear eye glasses you should purchase the special safety glasses made to wear over eye glasses.
b) Safety goggles cannot be worn in this lab.
c) It is okay to wear your regular eye glasses with plastic side shields on them.
d) You may remove your safety glasses if you are packing up your belongings at the end of the lab period.
e) It is okay to remove your safety glasses if the fume hood sash is protecting your face.
a
Which of the following fires can be put out using water? (choose all of the correct answers)
a) Sodium
b) Paper
c) Wood
d) Electrical
e) Organic solvents (flammable liquids)
b and c
If the fire alarm sounds, what three things do you need to turn off before you leave the building? (choose all of the correct answers)
a) Electrical instruments, such as stirring plates
b) Gas valve
c) Air valve
d) Nitrogen valve
e) Water
a, b, and e
Which of the following statements regarding handling glassware in the lab is true?
a) When attaching tubing to a water condenser you should apply generous pressure with no twisting.
b) To remove tubing from a water condenser you should gently pull on it while using a twisting motion.
c) When applying a filter adapter to a Buchner funnel you should use a forceful, twisting motion.
d) Broken glass can be wiped up using a paper towel and disposed in the solid waste.
e) When attaching tubing to a water condenser you can lubricate the tubing with grease to make it easier.
b
Which of the following statements regarding handling glassware in the lab is false?
a) When attaching tubing to a water condenser you should lubricate the tubing with acetone or water to make it easier.
b) To remove tubing from a water condenser you should gently pull it while using a twisting motion.
c) Broken glass should be cleaned up using a broom and dustpan and disposed in the glass waste.
d) When applying a filter adapter to a Buchner funnel you should apply generous pressure while twisting.
e) When applying a filter adapter to a Buchner funnel you should gently push the adapter on with no twisting.
d
Which of the following statements regarding handling glassware in the lab is true?
a) When applying a filter adapter to a Buchner funnel you should push the adapter on with force.
b) When attaching tubing to a water condenser you should lubricate the tubing with acetone or water to make it easier.
c) To remove tubing from a water condenser you should pull it straight off.
d) When applying a filter adapter to a Buchner funnel you should apply generous pressure while twisting.
e) Broken glass can be wiped up using a paper towel and disposed in the solid waste.
b
Which of the following statements regarding handling chemicals is/are true? (Select all that apply.)
a) All chemical waste can go into the waste bottles found in the sink.
b) Flammable liquids can be heated using an open flame.
c) Unlabelled chemicals must not be used
d) If you spill concentrated acid on the floor it can be neutralized using sodium bicarbonate.
e) When diluting acid you should slowly add water into acid that is cooled in an ice-water bath.
c and d
Which of the following statements regarding handling chemicals is/are true? (Select all that apply.)
a) When diluting acid you should slowly add acid to water.
b) If you spill a small amount of concentrated acid on the floor, you should neutralize it with sodium bicarbonate.
c) Flammable liquids can be heated using an open flame.
d) All chemical waste can go into the yellow waste container in the waste fume hood.
e) Unlabelled chemicals must not be used.
a, b, and e
Which of the following statements regarding handling chemicals is/are false? (Select all that apply.)
a) When making an acidic solution, you should slowly add acid to water.
b) If you spill concentrated acid on the floor you can neutralize it with 6 M NaOH(aq).
c) Flammable liquids can be heated using a heating/stir plate.
d) All chemical waste can go into the waste bottles found in the sink.
e) Unlabelled chemicals must not be used.
b and d
Which of the following statements regarding handling chemicals in the lab is false?
a) Large amounts of acid or base spilled in the lab can be neutralized with sodium bicarbonate.
b) When diluting acid you should constantly be agitating the mixture.
c) After chemicals are obtained from the front of the lab, they should be covered and taken directly to your bench top.
d) You should only get the amount of chemicals you need, avoiding excess waste.
c
Which of the following statements about precautions you should take in an organic chemistry lab is false?
a) Most chemicals are toxic by inhalation and should be kept in a fume hood as much as possible.
b) The fume hood sash protects you from inhalation of fumes and splatters to your face and neck.
c) Since you are working in a fume hood and wearing safety glasses the fume hood sash can be at any height.
d) Safety glasses should be worn at all times when in an organic chemistry lab.
e) Most chemicals in the lab are corrosive so you should always protect your skin with a lab coat and closed-toe shoes.
c
Which of the following statements regarding precautions in the organic laboratory is false?
a) If you are unsure about the set-up of an experiment, then ask a TA for help.
b) If the fire alarm sounds turn off all gas lines, electricity and water before leaving the building.
c) All chemicals in the organic lab should be handled carefully and treated as potentially hazardous.
d) If a chemical contacts your skin, flush the affected area with water for at least 5 minutes.
e) All reactions must be performed in the fume hood.
d
Which of the following statements about precautions you should take in an organic chemistry lab is true?
a) Some chemicals in the lab are corrosive so you only wear a lab coat and closed-toe shoes during experiments that corrosive chemicals are used.
b) Wearing safety glasses and working in a fume hood with the fume hood sash at the proper height acts as protection for your face, eyes and neck.
c) Most chemicals are not toxic by inhalation and only need to be kept in a fume hood when instructed to do so.
d) Safety glasses do not need to be worn when you are packing up your things and leaving the lab.
e) The fume hood sash only protects you from chemical splatters to your face.
b
Which of the following statements regarding precautions in the organic laboratory is true?
a) If you are unsure about the set-up of an experiment, then ask your friend for help.
b) If the fire alarm sounds, then only gas lines need to be turned off to avoid an explosion.
c) Not all reactions need to be performed in the fume hood.
d) If a chemical contacts your skin, flush the affected area with water for at least 15 minutes.
d
You are unsure about an aspect of the experiment you are doing, what should you do? (Select all that apply.)
a) Ask your friend what they did and do the same.
b) Refer to your plan of action, maybe you missed something.
c) Stop the experiment and leave the lab, organic chemistry is just not for you.
d) Stop and think about what you are trying to achieve, maybe you can answer your own question.
e) Go with your instincts and continue.
f) Ask a TA or the lab instructor for help.
g) Read the lab manual over and over again until you have an idea.
b, d, and f
It does not matter if you clean glassware with water or acetone first, only that you cleaned it using both solvents.
True or False
False
Cleaning your glassware with acetone after cleaning it with water is ideal since acetone helps remove residual water.
True or False
True
You should rinse glassware with acetone after you rinse with water because the acetone carries away residual water as it evaporates.
True or False
True
Since the products made in this lab are organic (and thus organic soluble), all reaction glassware should be rinsed with acetone first after a reaction.
True or False
True
After a reaction that produces an organic compound, you should always rinse the reaction glassware with acetone first. Not all of these rinsings can go directly into the acetone waste container, so always refer to the waste information at the end of the experimental procedure.
True or False
True
After a reaction that produces an organic compound, you should always rinse the reaction glassware with water first. All of such rinsings can go directly into the aqueous waste container.
True or False
False
Since the products made in this lab are organic (and thus organic soluble), all reaction glassware should be rinsed with water first after a reaction.
True or False
False
Which of the following statements regarding measuring out solid or liquid reagents is/are false? (Select all that apply.)
a) First, use a weighing paper to measure out a solid, then add the solid to the reaction glassware.
b) Reagents requiring an accurate volume will be obtained using a dispensing pump or autopipette.
c) If the volume required is greater than 1 mL, most solvents can be measured to the approximate volume using a graduated cylinder.
d) Solid reagents can be weighed out directly into glassware
e) If high-accuracy is desired; the required volume of a liquid limiting reagent can be measured using a graduated cylinder.
d and e
Which of the following statements regarding measuring out solid or liquid reagents is/are true? (Select all that apply.)
a) If the volume required is greater than 1 mL, most solvents can be measured to the approximate volume using a graduated cylinder.
b) Reagents requiring an accurate volume will be obtained using a dispensing pump or autopipette.
c) First, use a weighing paper to measure out a solid, then add the solid to the reaction glassware.
d) Solid reagents can be weighed out directly into glassware.
e) If high-accuracy is desired; the required volume of a liquid limiting reagent can be measured using a graduated cylinder.
a, b, and c
Which of the following statements regarding transferring reactions mixtures or dry solutions is/are true? (select all that apply)
a) When transferring a dried organic solution into a round bottom flask you should always rinse the reaction flask with an organic solvent and deionized water.
b) When transferring a dried organic solution into a round bottom flask you should never rinse the reaction flask with deionized water, only organic solvent.
c) You always want to try and transfer as much material as possible by rinsing with the appropriate organic solvent, and sometimes water, to maximize the yield.
d) When transferring a reaction mixture to a separatory funnel you should always rinse the reaction flask with an organic solvent and deionized water.
e) When transferring a reaction mixture to a separatory funnel you should never rinse the reaction flask with deionized water, only organic solvent.
b, c, d
Which of the following statements regarding transferring reactions mixtures or dry solutions is/are false? (select all that apply)
a) When transferring a dried organic solution into a round bottom flask you should always rinse the reaction flask with an organic solvent and deionized water.
b) When transferring a reaction mixture to a separatory funnel you should always rinse the reaction flask with an organic solvent and deionized water.
c) When transferring a dried organic solution into a round bottom flask you should never rinse the reaction flask with deionized water, only organic solvent.
d) You always want to try and transfer as much material as possible by rinsing with the appropriate organic solvent, and sometimes water, to maximize the yield.
e) When transferring a reaction mixture to a separatory funnel you should never rinse the reaction flask with deionized water, only organic solvent.
a and e
To test the pH of a solution, stir the solution with a glass rod, then dip the pH paper into the mixture. Compare the colour on the pH paper to the colour on the chart to determine the pH of the solution.
True or False
False
To test the pH of a solution, simply dip the pH paper into the middle of the solution. Compare the colour on the pH paper to the colour on the chart to determine the pH of the solution.
True or False
False
To test the pH of a solution, stir the solution with a glass rod and dab some of the mixture onto pH paper. Compare the colour on the pH paper to the colour on the chart to determine the pH of the solution.
True or False
True
A reagent has a melting point of -95 °C and a boiling point of 111 °C. Will it be a solid, liquid or gas under ambient conditions?
a) Solid
b) Liquid
c) Gas
b
A reagent has a boiling point of -78°C. Will it be a solid, liquid or gas under ambient conditions?
a) Solid
b) Liquid
c) Gas
c
A reagent has a melting point of 122 °C and a boiling point of 250 °C. Will it be a solid, liquid or gas under ambient conditions?
a) Solid
b) Liquid
c) Gas
a
Mixing hexane and ethyl acetate forms a homogenous mixture.
True or False
True (white on chart = miscible/homogenous)
When two liquids are not miscible, what property must you compare to know which will be the top layer and which will be the bottom layer?
a) Boiling point
b) Density
c) Molecular weight
d) Solubility
b

What functional group is this?
alcohol
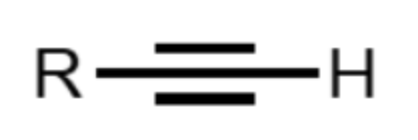
What functional group is this?
alkyne
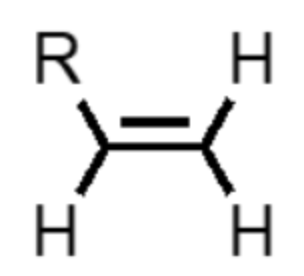
What functional group is this?
alkene
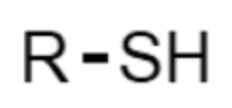
What functional group is this?
thiol
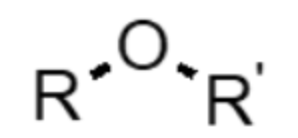
What functional group is this?
ether
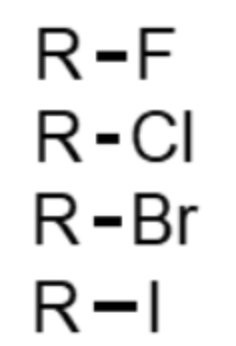
What functional group is this (R-X)?
alkyl halide
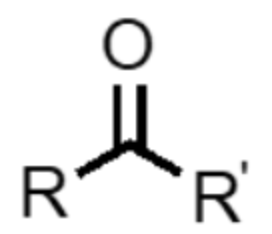
What functional group is this?
ketone
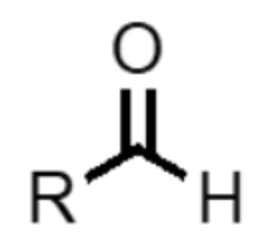
What functional group is this?
aldehyde
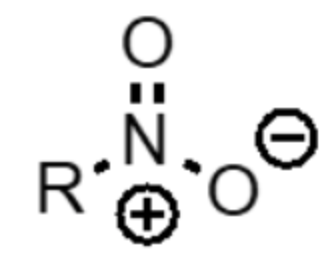
What functional group is this?
nitro
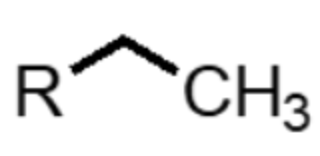
What functional group is this?
alkane
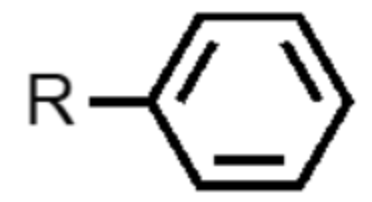
What functional group is this?
phenyl
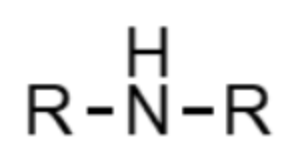
What functional group is this?
amine
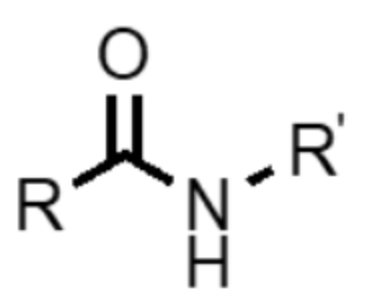
What functional group is this?
amide
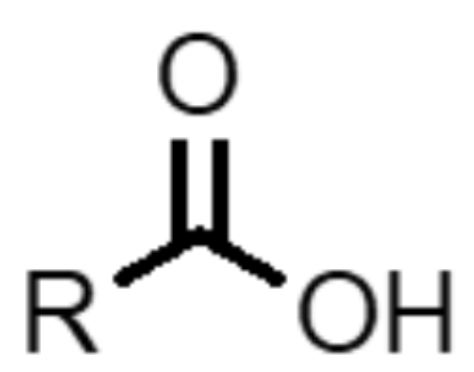
What functional group is this?
carboxylic acid
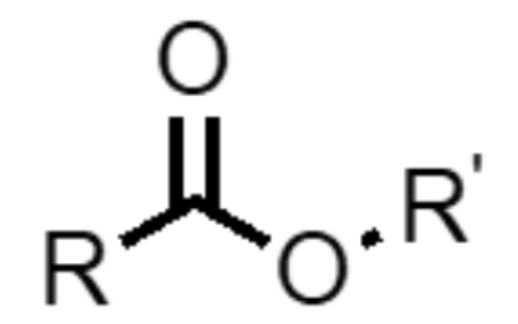
What functional group is this?
ester
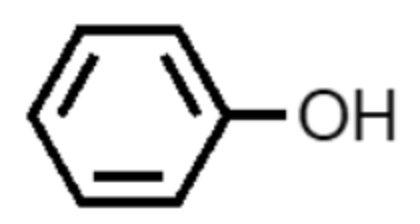
What functional group is this?
phenol

What functional group is this?
nitrile
Which solvent mixture is more polar?
a) 40% ethyl acetate in hexane
b) 10% ethyl acetate in hexane
c) 50% hexane in ethyl acetate
d) 20% hexane in ethyl acetate
d
Which solvent mixture is less polar?
a) 10% ethyl acetate in hexane
b) 40% ethyl acetate in hexane
c) 20% hexane in ethyl acetate
d) 50% hexane in ethyl acetate
a
Which of the following binary mixtures acceptable as TLC mobile phases? Select all that apply.
a) Dimethylformamide/methanol
b) Methanol/hexane
c) Diethyl ether/dichloromethane
d) Acetone/water
e) Acetone/dichloromethane
f) Ethyl acetate/hexane
g) Ethyl acetate/water
c, e, and f
What would you see on the TLC plate if a low concentration sample was spotted on the plate?
a) Multiple spots
b) Incorrect RF
c) Pale or invisible spots
d) Streaking spots
c
What would happen if you let the TLC plate run for too long and the solvent front reaches the top of the plate?
a) Streaking spots
b) Incorrect RF
c) Pale or invisible spots
d) Multiple spots
b
What would you see on the TLC plate if a high concentration sample was spotted on the plate?
a) Multiple spots
b) Incorrect RF
c) Pale or invisible spots
d) Streaking spots
d
What could happen if the mobile phase is too non-polar?
a) Multiple spots
b) Streaking spots
c) All spots together at bottom of plate
d) Incorrect RF
e) All spots together at top of plate
c
What could happen if the mobile phase is too polar?
a) All spots together at bottom of plate
b) Incorrect RF
c) Multiple spots
d) All spots together at top of plate
e) Streaking spots
d
Which of the following relating to distillation are false? (Check all that apply).
a) The boiling point of the distilled liquid is the temperature of the hot plate.
b) Distillation removes the lowest bp liquid from the flask/vial.
c) Distillation flasks can be distilled to dryness.
a and c
Select all the statements that are true about distillation
a) Add the sand after the flask is placed in the heating block
b) You need to turn on the water after the tubing is attached to the condenser
c) The liquid to be distilled should be added before the distillation glassware is assembled
d) You should clamp the Claisen head fro greater stability
a, b, and c
How will you identify which of the solvents the dye was dissolved in?
a) By looking at the colour of the distillate.
b) By comparing the measured boiling point to the literature values.
c) By searching solvents used in the textile industry online.
d) By smelling the distillate.
b
What would happen if the filter paper is not wetted prior to vacuum filtration?
a) The filtration would be slower
b) The solid would stay ‘wet’
c) The solid could escape to the vacuum flask
d) The filter paper would clog
c
Which of the following regarding vacuum filtration is incorrect?
a) Mixtures should be quantitatively transferred into the funnel
b) The filtrate can be disposed of in any waste container
c) Filter paper is wet with solvent before adding solution
d) The flask should be clamped
b
Which conditions allow recrystallization to be successful? (Select all that apply.)
a) Desired product and impurity precipitate out once cooled.
b) Desired product and impurity remains in solution once cooled.
c) Desired product remains in solution, and impurity precipitates out once cooled.
d) Desired product precipitates out once cooled, and impurity remains in solution.
c and d
Which statement(s) about recrystallization are true? (Select all that apply.)
a) The solution should not be disturbed while cooling
b) The smallest Erlenmeyer flask possible should be selected for boiling solvent
c) A large amount of solvent is used
d) Recrystallization removes impurities
e) Only one solvent is needed to recrystallize any sample
a and d
Given that compound A is more soluble in a non-polar organic solvent, and compound B is more soluble in water, which compound would you predict to be the more polar compound?
a) Compound A
b) Compound B
b
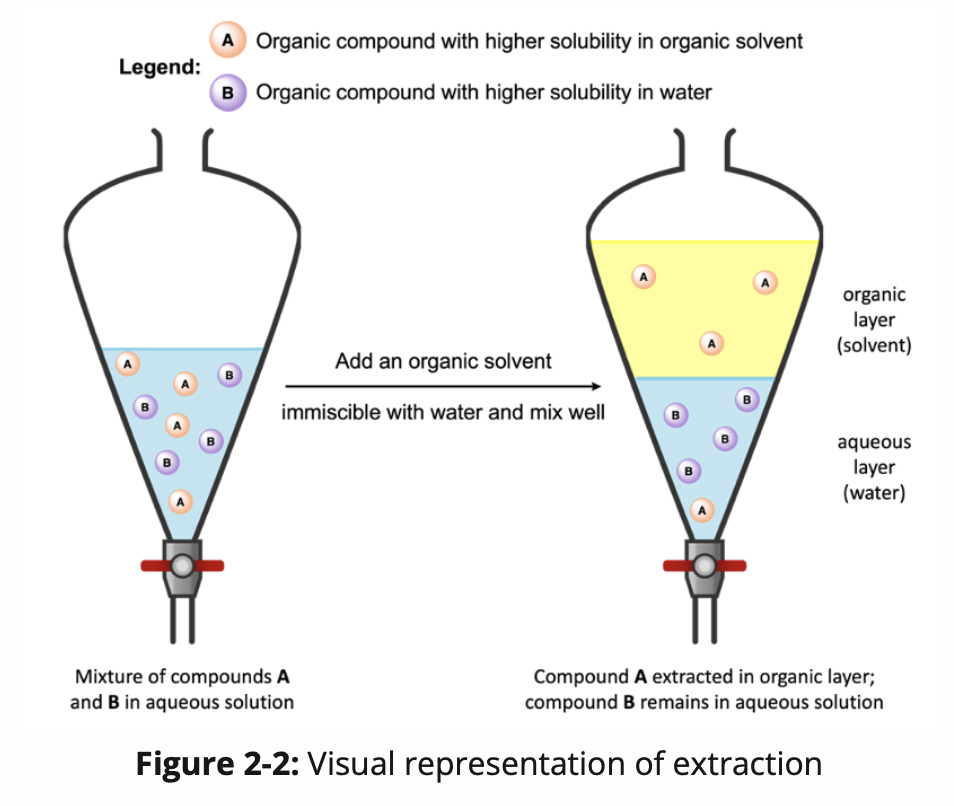
For the visualization shown in Figure 2-2, which of the following liquids would you expect to have a higher density?
a) Water
b) Organic solvent
a
You are doings a liquid-liquid extraction using water and acetonitrile, which layer is on top?
a) Water
b) Acetonitrile
c) Neither, they are miscible
c
Which of the following solvents would be generally acceptable for liquid-liquid extraction?
a) Dichloromethane
b) Diethyl ether
c) Isopropanol
d) Ethyl acetate (ethyl ethanoate)
e) Toluene
f) Triethylamine
a, b, d, and e
Which of the pieces of data will allow you to identify if your solid was acetaminophen, benzoic acid, acetanilide or vanillin?
a) Solubility
b) Yield
c) Appearance of crystals
d) Melting point
e) Boiling point
d
You are doing a liquid-liquid extraction using 3 M NaOH and diethyl ether, which layer is on top?
a) 3M NaOH
b) Neither, they are miscible
c) Diethyl ether
c
What is the definition of ‘hygroscopic’?
a) Absorbs water
b) Does not contain water
c) Insoluble in water
d) Soluble in water
a
What is the definition of ‘anhydrous’?
a) Absorbs water
b) Soluble in water
c) Does not contain water
d) Insoluble in water
c
Should you rinse the Erlenmeyer after decanting the solution into a round bottom flask?
Yes or No
Yes
What solvent should you use to rinse the Erlenmeyer?
a) Ethanol
b) None
c) Dichloromethane
d) Same solvent as bulk of the organic solution
e) Water
Solutions should be dried before rotary evaporation.
True or False
True
You forget to close the pressure valve of the rotavap. What potential problem could you have?
a) The evaporation would be very slow
b) The solvent could ‘bump’
c) The flask could fall in water bath
d) The rotavap could explode
c
Is caffeine a non-ionizable (neutral), acidic or basic compound?
a) Basic
b) Acidic
c) Non-ionizable (neutral)
a
What size of beaker should you use to make this solution? It should not be more than half-full!
a) 50 mL
b) 100 mL
c) 250 mL
d) 400 mL
e) 600 mL
c
What is the definition of an emulsion?
a) Liquids that cannot mix in any ratio
b) A spherical aggregation of molecules with a polar end and a hydrophobic chain
c) Fine dispersion of droplets of liquids that are miscible
d) Fine dispersion of droplets of liquids that are not miscible
e) Liquids that can mix together in any ratio
d
SN2 reactions will always affect the absolute configuration (ie. R or S) of the electrophile.
True or False
False
SN2 reactions will sometimes affect the absolute configuration (ie. R or S) of the nucleophile.
True or False
False
In a balanced chemical equation, we put equivalents as stoichiometric coefficients.
True or False
False
The limiting reagent is always the reactant with the quantity set as one equivalent.
True or False
False
Equivalents used in a reaction are always greater than the stoichiometric coefficients.
True or False
False
If you weighed 1 g of Tylenol powder, would the starting amount of acetaminophen be 1 g?
Yes or No
No
How would the theoretical yield be affected if you used 1 g of Tylenol powder instead of using all the powder from the two tablets provided to you?
a) The theoretical yield would increase
b) The theoretical yield would decrease
b
How will you know that your reaction is complete by TLC?
a) By comparing the measured Rf values of the spots in the RM lane to the literature values for 4-butyoxyacetanilide
b) If there is only one spot in the RM lane, the reaction is complete.
c) By comparing the measured Rf values of the spots in the RM lane to the literature values for acetaminophen.
d) If no spot in the RM lane travelled the same distance as the spot in the SM lane, the reaction is complete.
d
When comparing to the stoichiometric equivalents, which reagents would be considered in excess? Select all that apply.
a) Acetaminophen
b) 1-Iodobutane
c) NaOH in EtOH
d) 4-butoxyacetanilide
b and c