Unit 6 Bonding >
6.1 Predicting Bonding Types
Bonding theories are used by researchers to predict how atoms are bonded to form compounds, and what combinations of atoms form them. They are the reason why water is H2O instead of H3O and explain the shapes that determine an element's properties. You can also predict an element’s bond type through its different properties. Three bonds are:
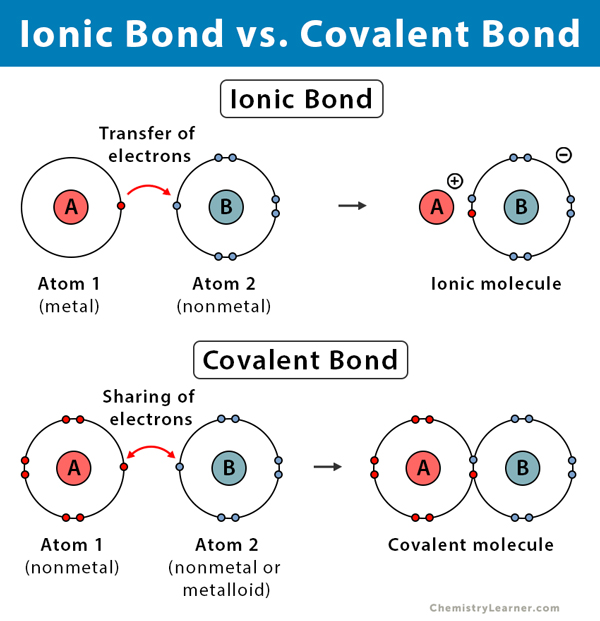
Ionic Bond (Metals and nonmetals) Covalent Bond*(Nonmetals and nonmetals)* Metallic Bond*(Metals and metals)* | ||
Conductive (in water) | No conductivity | Yes conductivity |
Transfers electrons | Shares electrons | Electron sea* |
High melting/boiling point | Low melting/boiling point | High melting/boiling point |
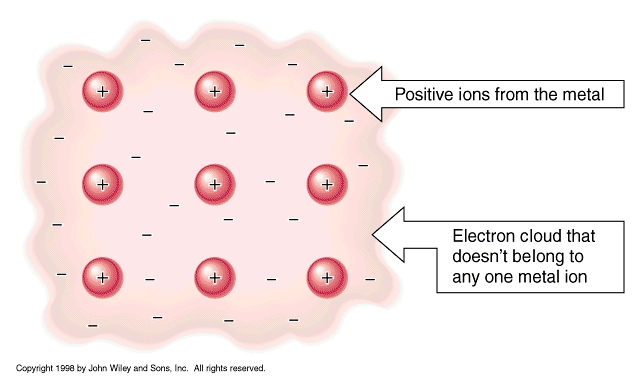
*Electron Sea- Since metals are good conductors, their electrons are easily lost. When metal atoms near one another lose their outer shell electrons, they arrange themselves in a “sea” of electrons which can move freely instead of orbiting around their respective atoms.
Using electronegativity to identify bond types:
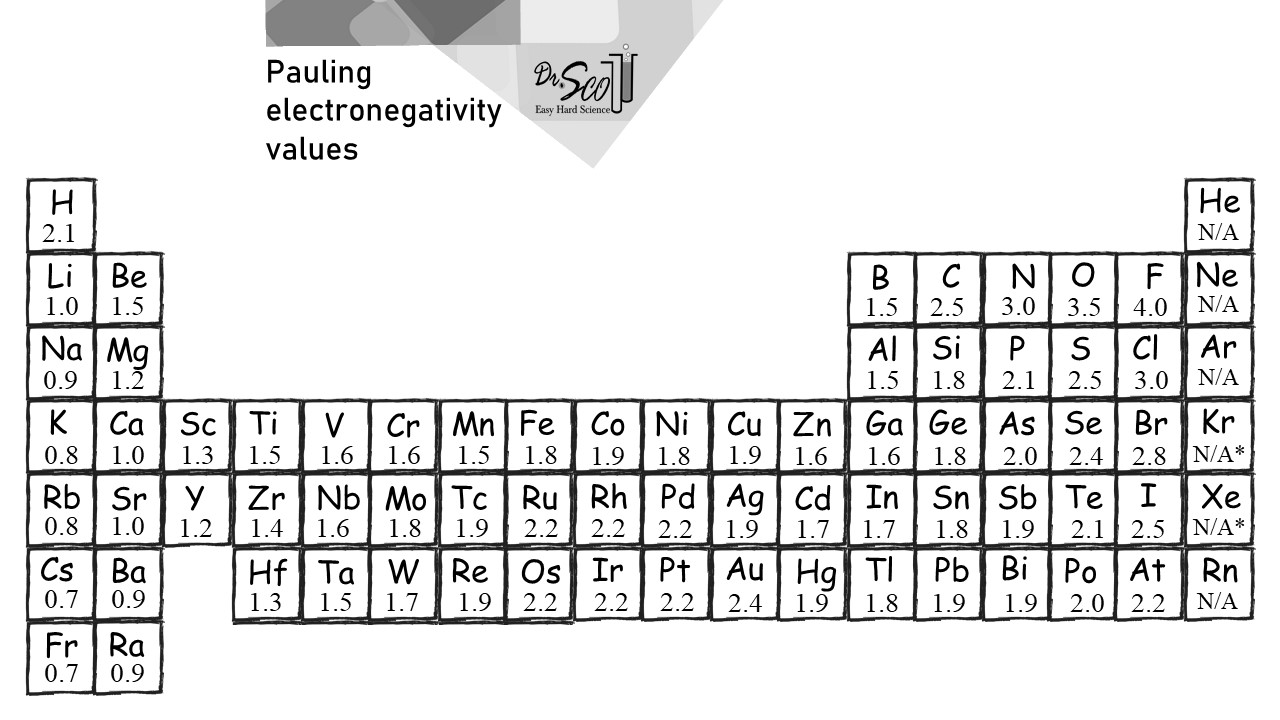
\Electronegativity is the tendency for atoms to attract electrons to themselves. As we go up each group of the periodic table, electronegativity increases, this is the same as you go down each row. We can use these values to identify the bond types between two elements by subtracting the values from one another and categorizing them.
Ionic Difference > 1.7 | |
Covalent | 0.4 < Difference < 1.7 |
Metallic / Covalent | Difference < 0.4 |
Example:
Rubidium (0.8) and chlorine (3.0) subtracted from one another is 2.2. Since 2.2 is greater than 1.7, the bond is ionic.
Another method is to use the categories on the periodic table
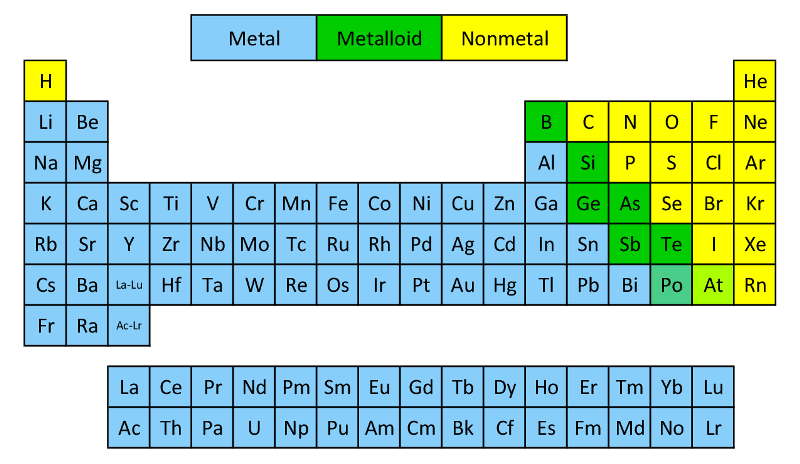
As you can see, each element has its own category: metal, metalloid, or nonmetal. If we recall, ionic is a bond between metals and nonmetals, covalent is a bond between nonmetals, and metallic is a bond between metals. If we use the rubidium and chlorine example again, we can see that rubidium is metal while chlorine is a nonmetal. This means that the bond is ionic.
6.2 Lewis Structures
The Lewis theory is named after Gilbert N. Lewis, the American chemist who created it. We create Lewis structures to represent molecules, using dots as their electrons. This theory predicts a molecule's stability, as well as its appearance.
Remember that the valence electron quantity for any element (excluding helium) is equivalent to the element's group number.
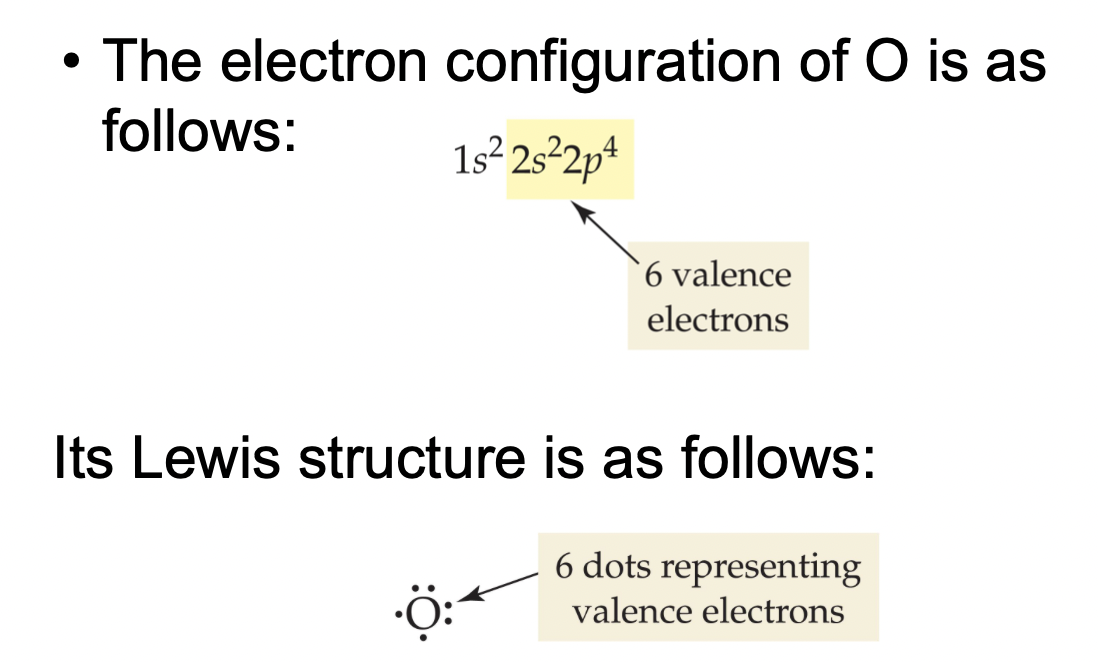
With this method, we can easily identify the number of valence electrons for each element.
Practice Problem
Draw the Lewis Structure for the element Phosphorous.
Hint: Phosphorous is in Group 5A of the Periodic table
Answer:
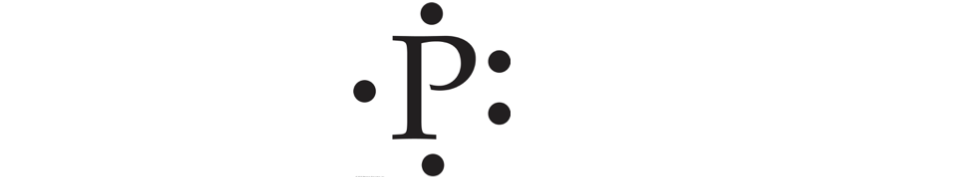
Showing Bonds in Lewis Structures
Ionic bonds consist of a nonmetal and a metal. Electrons are transferred from metals to nonmetals, causing the metal to become a cation and the nonmetal into an anion.
Remember that elements are trying to gain stability through an 8-electron outer shell, which is why the electron in this example is transferred from potassium and chlorine. The charge for potassium becomes positive because it has lost an electron, while Chlorine became negatively charged(One way to think of is that when an electron is lost, the element should become more positive because the electron which was lost is negative).
How do you write formulas?
Sodium chloride- Na Cl <- base term for chloride (cation/metal first, nonmetal/anion second)
In ionic compounds, we use the simplest ratio of elements to represent their formula, for example, sodium chlorides ratio is 1 to 1 so
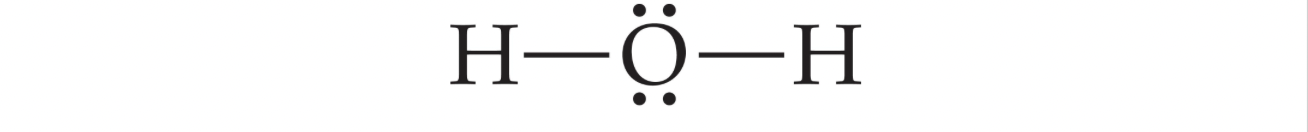
In Covalent bonds, elements share electrons to attain octets.
Shared electrons between two elements are called bonding pairs, and can be drawn with a dash instead of two dots:
Double Bonds are used when you don't have enough electrons to form an octet with a single bonding pair. We can convert a lone pair into another bond to make a double bond.
An example of this is between two oxygen atoms:

Triple Bonds are also used to help satisfy the octet rule between two atoms. For example, the bond between two nitrogen atoms:
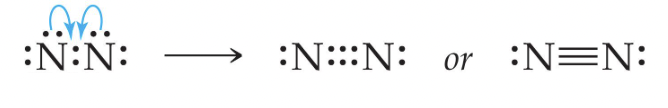
Diatomic molecules are elements with exactly two elements in them. They can either be between two different elements (CO) or the same (O2). The most common diatomic elements are: H2, O2, F2, Br2, I2, N2, Cl2
Bond Strength: Triple bond > Double bond > Single bond
Bond length: Single bond > Double bond > Triple bond
Resonance Structures
When creating a structure to represent molecules, you may come across equivalent bonds that both work. This means that for one formula, you may have 2 structures representing it.
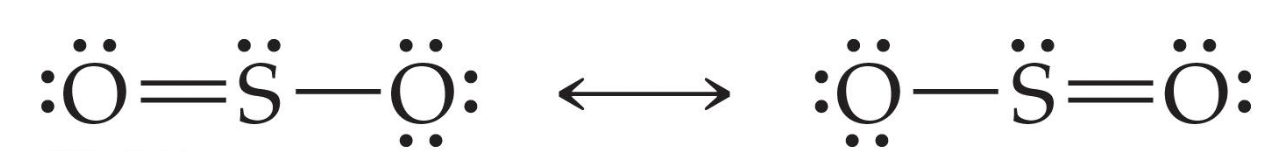
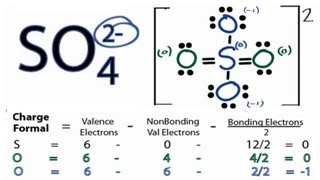
Step 1: Start by determining the number of electrons there are in total.
Both sulfur and oxygen have the same amount of electrons: 6, but we multiply oxygen by 4 due to how there are 4 of the same element. We also add 2, because of the superscript meaning that there are 2 extra electrons.
6 + 4(6) = 32 electrons
Step 2: We then format the structure, by having sulfur at the center and oxygen atoms surrounding it, and start placing pairs down
Step 3: We end up with this
6.3 Lewis Structures Exceptions
Helium
Helium is an exception because despite being in group 8A, it only has two electrons meaning it only has two paired dots on its Lewis structure.
The Octet Rule states that in chemical bonding, atoms will transfer or share electrons to obtain eight electrons in their outer shell. This means that a stable electron configuration typically contains eight electrons in its valence section. Though, three elements are the exception to this rule: hydrogen, lithium, and beryllium. These elements are stable with just two electrons in their outer shell which explains Helium’s lewis structure.
6.4 Polarity and Molecular Geometry
The Valence shell electron pair repulsion theory (VSEPR) is a model used to predict the shape of a compound with a nonmetal central atom. The theory states that electron pairs are negatively charged, so they will try and get as far apart from one another as possible. Electron pairs will arrange themselves to minimize repulsion, forming various shapes which we can determine with a configuration’s number of *charge centers, bonded and lone pairs.
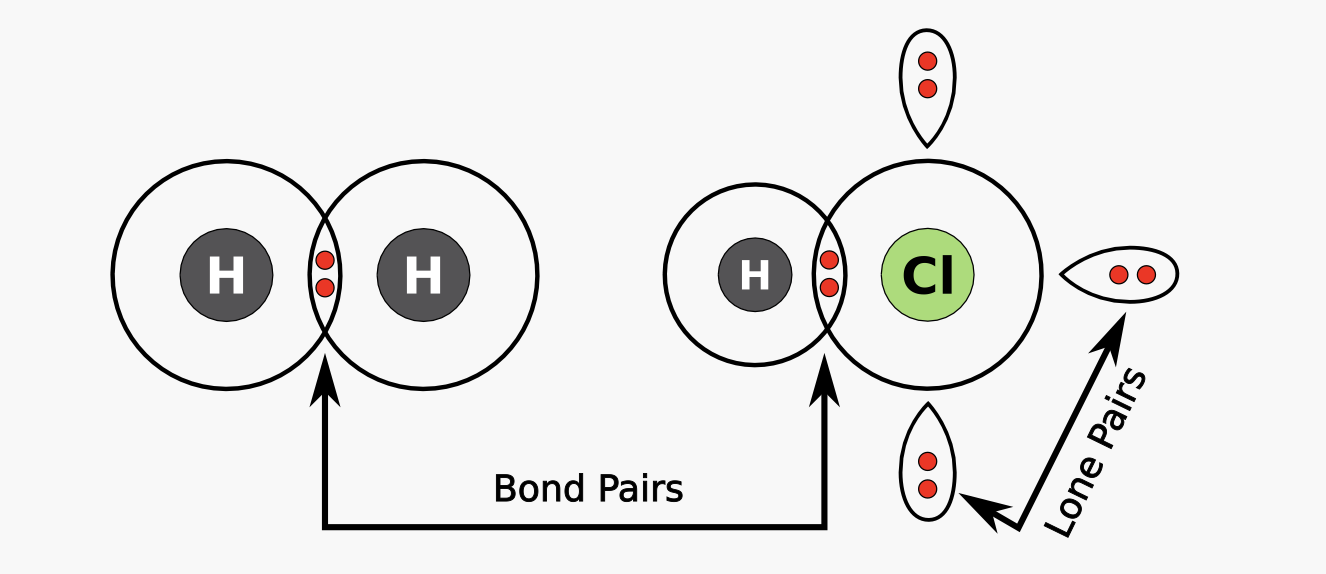
*A charge center is the number of bonded and lone pairs added up
Shape Charge Centers Bonded pairs Lone pairs Angle | ||||
Linear (CO2) | 2 | 2 | 0 | 180° |
Planar Trigonal (H2CO) | 3 | 3 | 0 | 120° |
Tetrahedral (CH4) | 4 | 4 | 0 | 109.5° |
Bent (H2O) / (SO2) | 4 / 3 | 2 / 2 | 2 / 1 | 109.5° / 120° |
Trigonal pyramidal(NH3) | 4 | 3 | 1 | 109.5° |
If there are 2 bonded pairs and at least 1 lone pair, then the shape is bent.
6.5 Polarity and Intermolecular
Oil and water being unable to mix is an example of polar (water) and nonpolar. In order molecule to be considered polar, you need at least two different elements, and the electronegativity difference must be greater than 0.4, but less than 1.7. You also need to molecule to have geometric asymmetry and also have *dipole moment. So essentially, polar molecules are both asymmetrical in electronegativity and geometry.
Tip: In a Lewis structure, if there is a lone pair, it is ALWAYS polar. If a structure is symmetric, it is NON-POLAR.
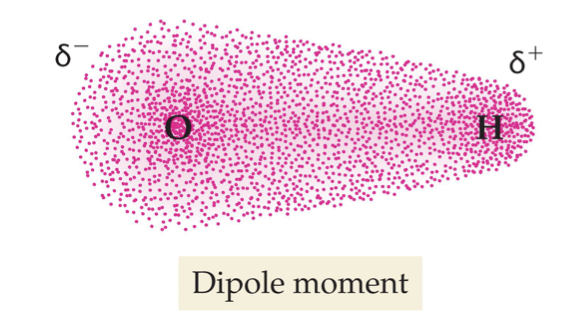
*Dipole moment-
A separation of the charge around the molecule into a more positive area and a more negative area / A separation of charge within the bond (uneven electron sharing)
Polar Covalent Bond Non-polar Covalent Bond Ionic Bond | ||
0.4 < difference <1.7 | 0 < difference <0.4 | difference > 1.7 |
6.6 Polarity Diagrams
Intramolecular
Forces that hold atoms together in a molecule: covalent, ionic, and metallic bonding
Intermolecular
Forces that exist between molecules: dipole-dipole, ionic-dipole, Hydrogen bond, dispersion force
Strongest to weakest= Ionic-dipole > H bond > dipole-dipole > dispersion force