Atomic structure
5.0(1)
Card Sorting
1/30
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
1
New cards
atom
smallest unit of an element that retains the properties of that element.
2
New cards
proton charge
positive
3
New cards
proton mass
1 amu (+)
4
New cards
what is inside the nucleus?
proton and neutron
5
New cards
neutron charge
neutral
6
New cards
neutron mass
1 amu (=)
7
New cards
electron charge
negative
8
New cards
mass of electron
no mass (≈ zero)
9
New cards
where is electron locacted
outside nucleus (electron cloud)
10
New cards
nucleus charge
positive
11
New cards
the nucleus is almost…
all the mass of the atom
12
New cards
electron cloud is where most of the…
volume is
13
New cards
atomic number
number of protons
14
New cards
what does the number of protons determine?
the element
15
New cards
atomic number
number of protons
16
New cards
Mass Number
sum of protons and neutrons
17
New cards
superscript of nuclear symbol
mass number
18
New cards
subscript of nuclear symbol
atomic number
19
New cards
Hyphen notation
the mass number is written with a hyphen after the element name
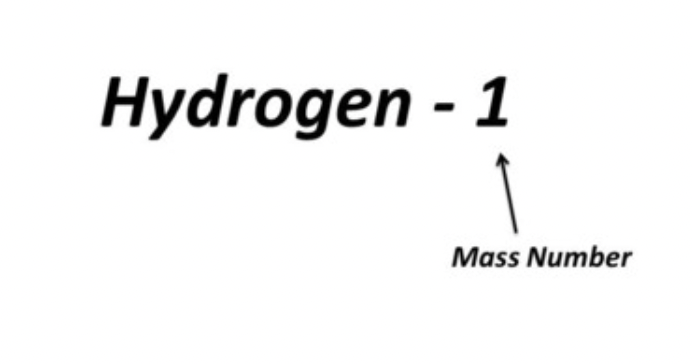
20
New cards
nuclear symbol
a type of shorthand notation that identifies the element (by symbol or atomic number) and the mass number of the element
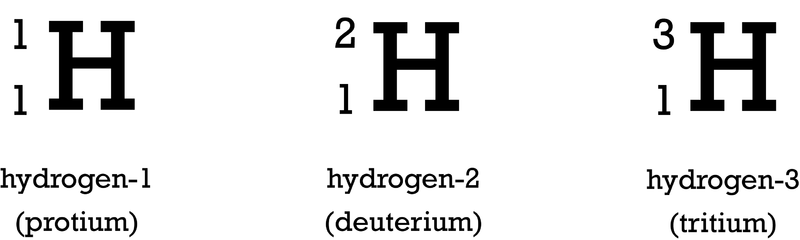
21
New cards
7
Li
3
atomic number?
Li
3
atomic number?
3
22
New cards
7
Li
3
mass number?
Li
3
mass number?
7
23
New cards
7
Li
3
number of protons?
Li
3
number of protons?
3 protons
24
New cards
7
Li
3
number of neutrons?
Li
3
number of neutrons?
4 neutrons
25
New cards
7
Li
3
number of electrons?
Li
3
number of electrons?
3 electrons
26
New cards
periodic table atomic number is a
whole number
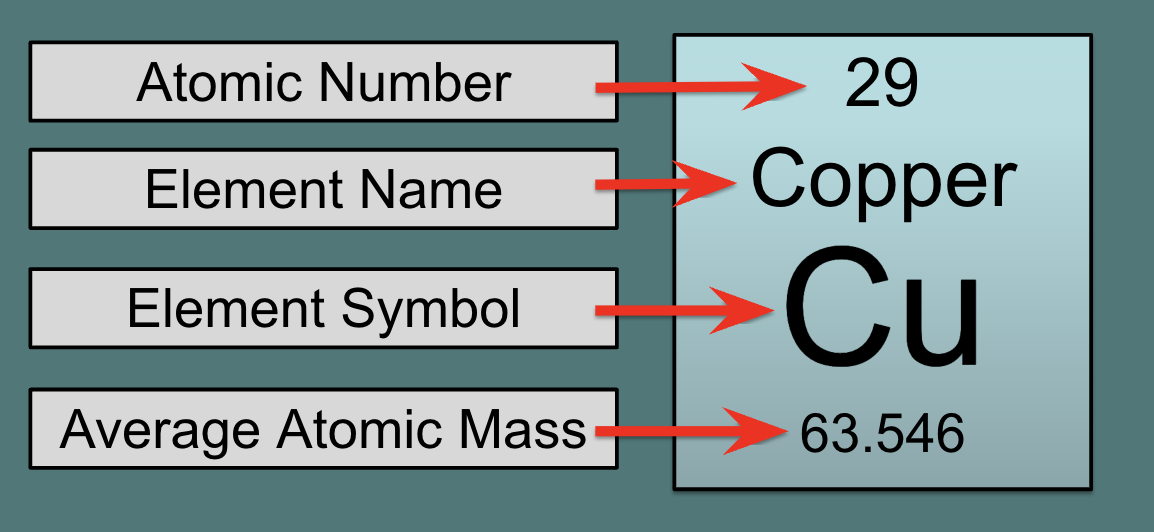
27
New cards
average atomic mass has ______ on the periodic table
decimals
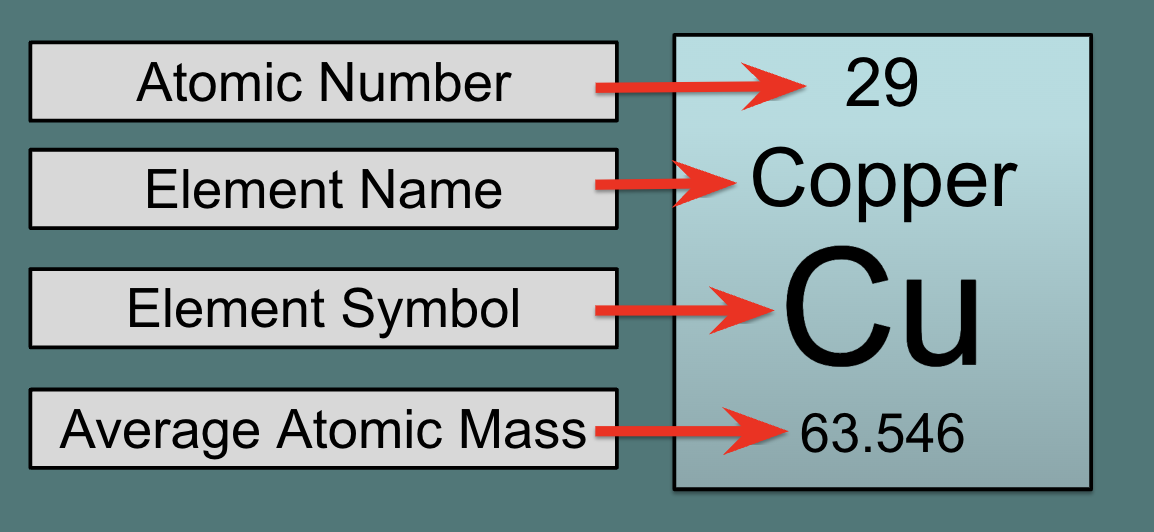
28
New cards

average atomic mass
79\.904 amu
29
New cards

mass number
80
30
New cards

atomic number
35
31
New cards

number of protons, neutrons, electrons (x, y, z)
35, 45, 35