Lecture 8: Sex Linkage
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Describe the various mechanisms in which organisms determine sex.
In some organisms, like humans, sex is determined by the X and Y sex chromosomes
Females are XX
Males are XY
In other organisms, like birds, butterflies, and some reptiles, sex is determined by the X and Z chromosomes
Females are ZW
Males are ZZ
In some organisms, such as turtles and alligators, sex is determined by the temperature at which eggs are incubated
The X and Y sex chromosomes pair during meiosis and segregate, but are not homologous — most genes on the X chromosome differ from genes on the Y chromosome, and this difference in the number of sex-linked alleles produces distinct patterns of inheritance in males and females.
Thomas Hunt Morgan’s fruit fly experiments
Morgan was breeding fruit flies when he noticed an interesting phenotype: some fruit flies had white instead of red eyes
Question: What is the genetic basis of the white-eyed phenotype?
Bred a white-eyed fly (ww) and a red-eyed (W+W+) fly
F1 generation (W+w) all had red eyes
Pattern of inheritance seemed to be Mendelian
Morgan expected a 3:1 ratio in F2 offspring
But instead, Morgan found that 100% of females were red-eyed, 50% of males were red-eyed, and 50% of males were white-eyed
Conclusion: The white-eyed phenotype in fruit flies was not transmitted through Mendelian patterns of inheritance.
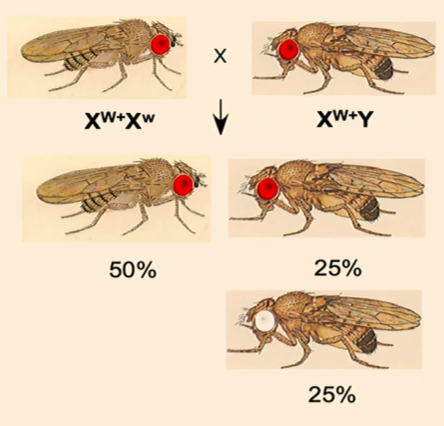
Fruit fly eye color is sex-linked, with red eyes being dominant and white eyes being recessive. If a heterozygous red-eyed female is crossed with a red-eyed male, what are the expected phenotypic ratios in the offspring?
Red-eyed female: XW+ Xw
Red-eyed male: XW+ Y
Punnett square ratios:
XW+ XW+ (red-eyed female)
XW+ Xw (red-eyed heterozygous female)
XW+ Y (red-eyed male)
Xw Y (white-eyed male)
Expected results: 100% red-eyed females, 50% red-eyed males, 50% white-eyed males
Describe the significance of Morgan’s experiments.
Morgan’s work showed that the gene for eye color in fruit flies was located on the X chromosome only, and that no homologous allele is present on the Y chromosome → proved that genes are inherited on chromosomes, supporting the chromosome theory of inheritance
Female organisms have cells with two X chromosomes (XX) — females can be homozygous or heterozygous for the eye-color allele
Male organisms have cells with one X and one Y chromosome (XY) — males cannot be homozygous or heterozygous because they only have 1 copy of the X chromosome
Males are hemizygous (only having one copy of a gene or chromosome) for X-linked loci
X chromosome
Contains genetic information essential for both males and females — at least one copy of this chromosome is required for proper cell functioning
Sex-determining region Y (SRY)
Also known as the male-determining gene; located on the Y chromosome
A single Y, even in the presence of several Xs, still produces a biological male phenotype
The absence of Y results in a biological female phenotype
XX with an insertion of the SRY gene on an X chromosome will result in…
A biological male phenotype
XO with an insertion of the SRY gene on an autosomal chromosome will result in…
A biological male phenotype
Karyotyping
A technique to visualize chromosomes — actively dividing cells are halted in metaphase by treatment with a mitotic spindle inhibitor, and chromosomes are arranged according to size
Turner syndrome
XO → biological female phenotype
Memorization trick: turner = “turn her" (biological female sex, two syllables)
Klinefelter syndrome
XXY, XXXY, XXXXY, XXYY → biological male phenotype
Memorization trick: Kline “felt her” (many Xs but biological male sex)
Poly-X females
XXX, XXXX, or more → biological female phenotype
Pseudoautosomal regions
The only regions where the X and Y chromosomes are homologous
Located at both tips of the X and Y chromosomes
Essential for X-Y chromosome pairing in meiosis in males
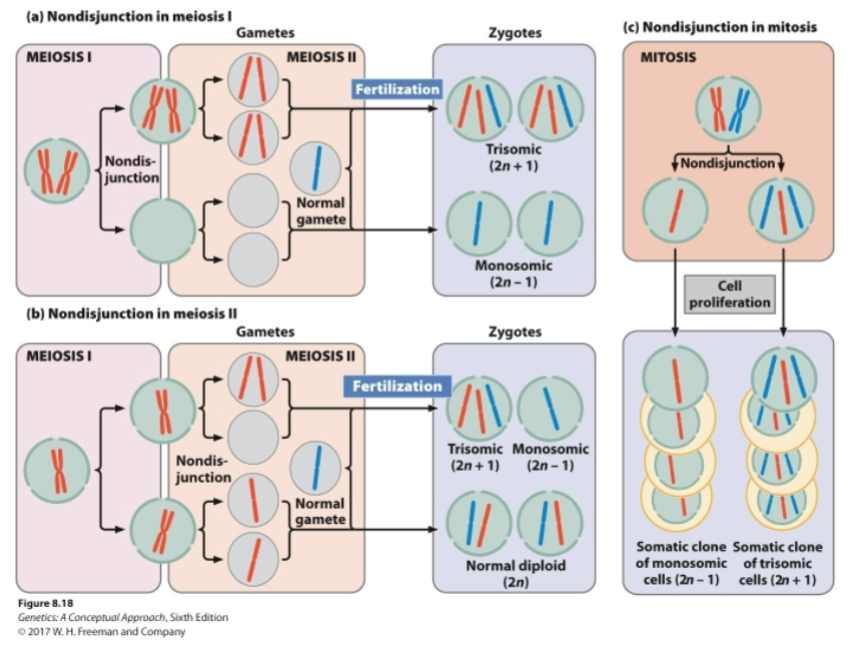
Define aneuploidy and explain how aneuploidy can occur because of errors in meiosis I, meiosis II, and mitosis.
A change in the number of individual chromosomes
Polyploidy
An increase in the number of sets of chromosome
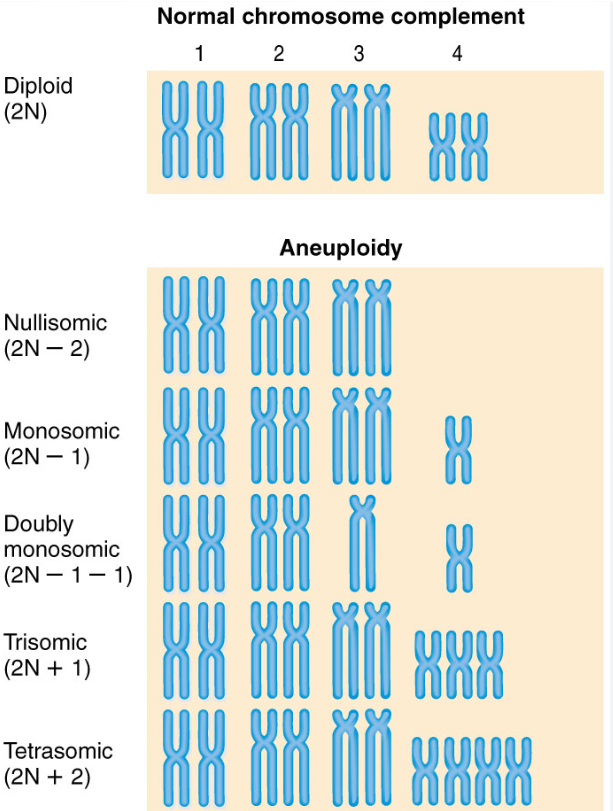
Describe the types of aneuploidy.
Nullisomy: loss of both members of a homologous chromosome pair (2n-2)
Tetrasomy: gain of a homologous chromosome pair (2n+2)
Monosomy: loss of a single chromosome (2n-1)
Trisomy: gain of a single chromosome (2n+1)
Explain the problem of XX-XY gene dosage.
In females, there are two copies of the X chromosome and two copies of each autosome, so the genes on the X chromosome and autosomes are in balance
In males, there is only one copy of the X chromosome and two copies of every autosome, so males are likely to produce lower amounts of proteins encoded by X-linked genes than of proteins encoded by autosomal gene
Some organisms have evolved mechanisms to overcome this problem and equalize the amounts of protein produced by the single X chromosome and two autosomes
Dosage compensation
Mechanisms to equalize the different amounts of protein produced by the single X chromosome and two autosomes in males
X-inactivation in females
An important mechanism to normalize X gene dosage between females (XX) and males (XY)
Within each female cell, one of the two X chromosomes is randomly inactivated
The inactivated X chromosome condenses into a Barr body
Because of X-inactivation, females are hemizygous at the cellular level for X-linked genes
In females that are heterozygous at an X-linked locus, 50% of the cells express one allele and 50% express the other allele — proteins encoded by both alleles are produced, but not within the same cell
The cells in an individual female are not identical with respect to expression of the genes on the X chromosome
Females are mosaics for the expression of X-linked genes!
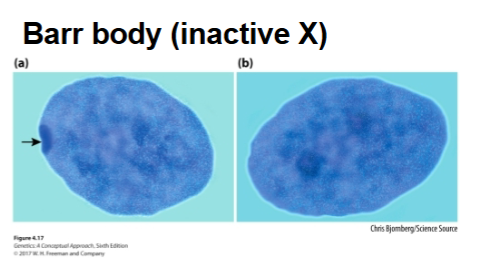
Random X-inactivation
Occurs early in development
After an X chromosome has become inactivated in a cell, it remains inactive in that cell and in all somatic cells that descend from that cell
Neighboring cells tend to have the same X chromosome inactivated, producing a patchy mosaic pattern in heterozygous females (ex. calico cats)
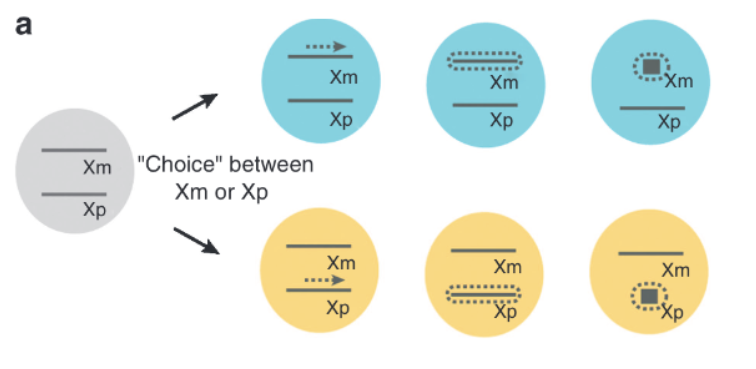
Describe the three reasons for non-random X-inactivation.
Random chance
Genetic bias — found in females heterozygous for a disease
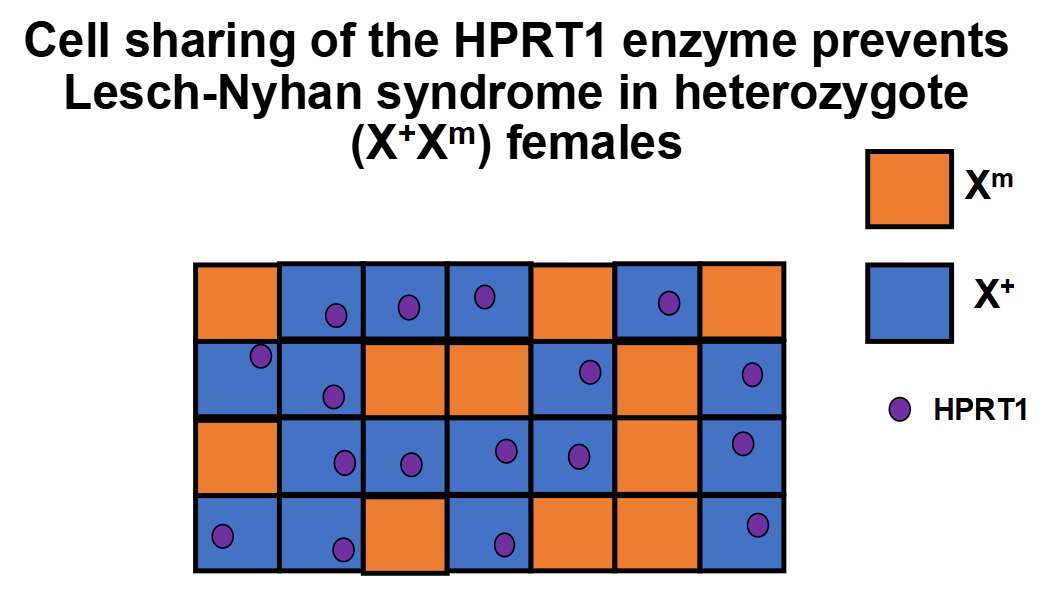
Example: Buildup of uric acid in Lesch-Nyhan syndrome due to mutations in the X-linked HPRT1 gene
Females heterozygous for this disease experience less severe effects due to cell sharing of the HPRT1 enzyme across gap junctions
Wild-type cells that express the HPRT1 enzyme are able to share the enzyme with mutant cells lacking it
This is an example of non-random X-inactivation due to genetic bias, where cells are more likely to inactivate the mutated X chromosome to facilitate sharing of the enzyme
Cellular selection — due to a growth advantage or disadvantage
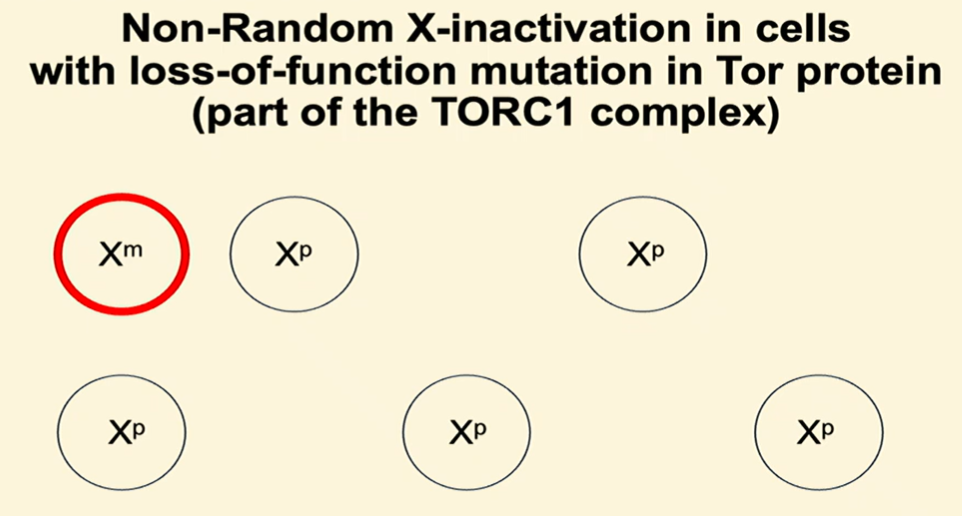
Example 1: Loss-of-function mutation in TORC1
TORC1 is a protein complex that is part of the G1 cell cycle checkpoint, responsible for sensing nutrient availability
If nutrients are limited, TORC1 will arrest the cell cycle in G1
If there is a mutation in TORC1 so that TORC1 is unable to sense nutrient availability, cells with the mutation will divide without adequate nutrients and die
If the mutated TORC1 is on the Xm chromosome, those cells will have a higher chance of dying, so there will be non-random X-inactivation where cells that inactivate the mutated Xm chromosome have a survival advantage over cells that inactivate the normal Xp chromosome
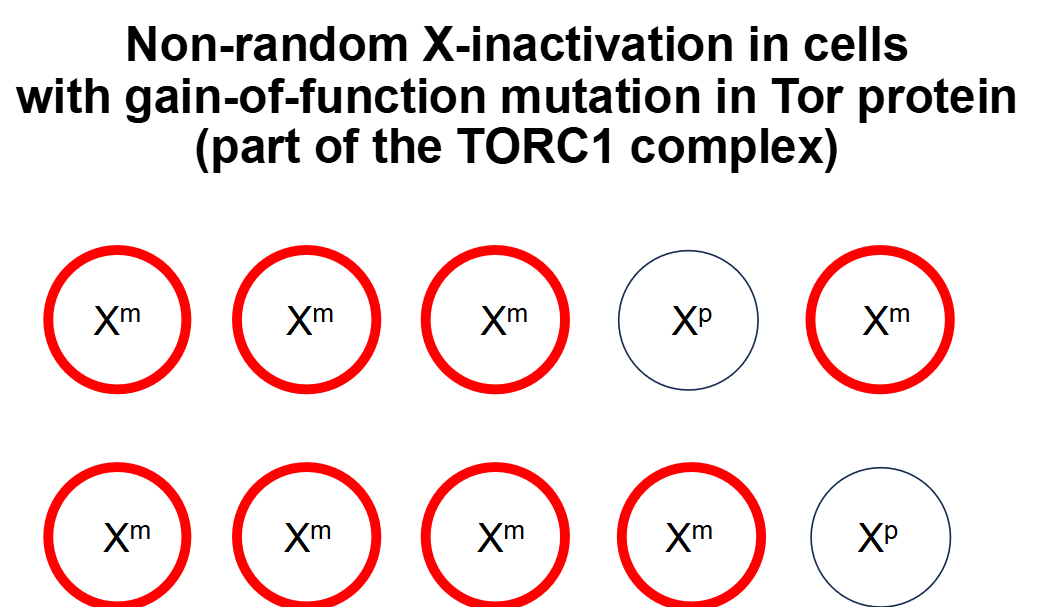
Example 2: Gain-of-function mutation in TORC1
A gain-of-function mutation in TORC1 will stimulate the cell cycle and result in increased rates of cell division for cells containing the mutated Xm chromosome — therefore, there will be non-random X-inactivation because cells containing the mutated Xm chromosome will outcompete cells containing the normal Xp chromosome

Explain the significance of biological females being genetic mosaics.
Females almost always have less severe manifestations of X-linked diseases, because heterozygous females are not homozygous for the pathogenic variant and contain both affected and unaffected cells
Variant cells expressing the deleterious allele receive sufficient levels of protein from cells expressing the normal allele to carry out essential metabolic functions → a way for females to ameliorate the effects of pathogenic variants