Chapter 7: Acids, bases and salts (copy)
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
acid
proton donors
pH below 7
H+ ions make the acidic
strong acid
an acid that completely dissociates in an aqueous solution, produce lots of H+ ions
example of strong acid, why is it a strong acid
hydrochloric acid
HCl (aq) gives H+ (aq) + Cl- (aq)
weak acid
an acid that partially dissociates in an aqueous solution, produce few H+ ions
example of weak acid, why is it a weak acid
ethanoic acid
CH3COOH (aq) reversibly gives H+ (aq) + CH3COO- (aq)
characteristic properties of acids: reaction with metals
acid + metal gives salt + hydrogen gas
characteristic properties of acids: reaction with bases
acid + base gives salt + water
characteristic properties of acids: reaction with carbonates
acid + carbonate gives salt + carbon dioxide + water
acids: effect on litmus
blue to red
acids: effect on thymolphthalein
stays colourless
acids: effect on methyl orange
orange to red
base
proton acceptors
oxides or hydroxides of metals
pH above 7
OH- ions make them basic
alkali
soluble bases
characteristic properties of bases: reaction with ammonium salts
base + ammonium salt gives salt + water + ammonia gas
bases: effect on litmus
red to blue
bases: effect on thymolphthalein
colourless to blue
bases: effect on methyl orange
orange to yellow
acid (aq) contains
H+ ions
base (aq) contains
OH- ions
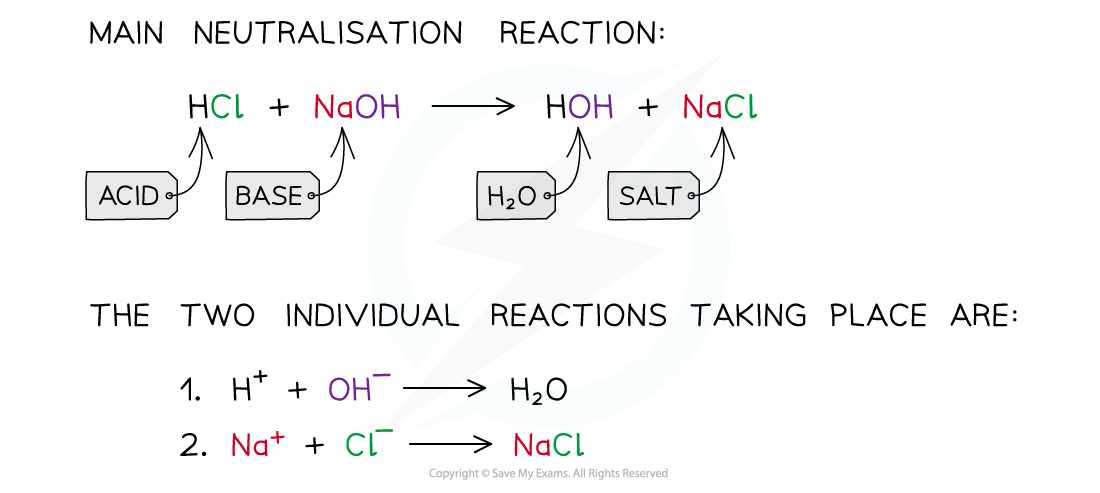
neutralisation reaction
neutralisation reaction occurs when an acid reacts with an alkali
H+ ions react with the OH– ions to produce water
e.g H+ (aq) + OH- (aq) gives H2O (l)
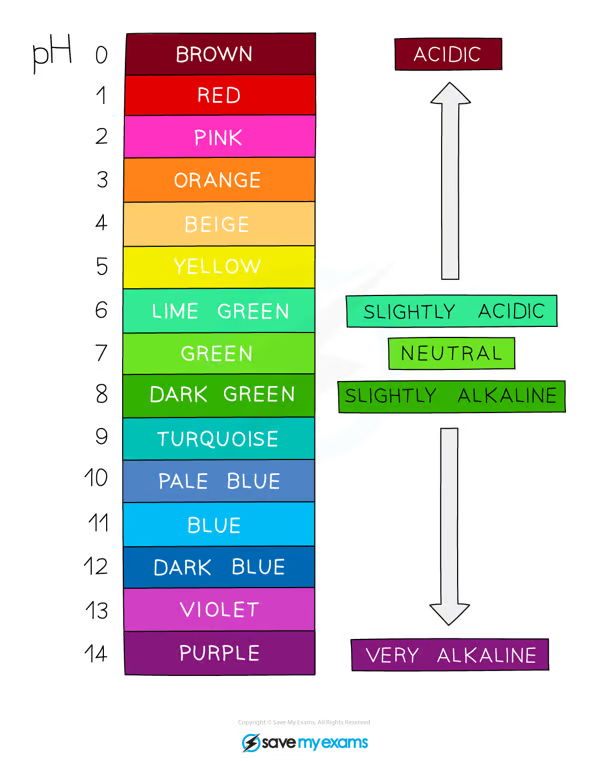
hydrogen ion concentration and universal indicator (colours and pH)
the higher the concentration of hydrogen ions, the stronger the acid, but the lower the pH
the lower the concentration of hydrogen ions in a solution, the weaker the acid, the higher the pH
(applied to hydroxide ions as well)
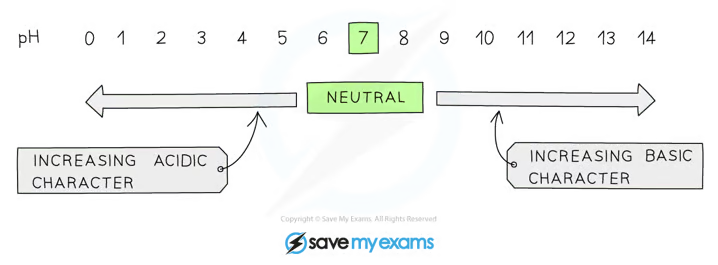
pH
pH is a measure of the concentration of H+ ions in solution, but they have an inverse relationship
lower the pH then the more acidic the solution is
The higher the pH then the more alkaline the solution is
A solution with a pH of 7, such as water or distilled water, is described as being neutral
pH scale is logarithmic, meaning that each change of 1 on the scale represents a change in concentration by a factor of 10
oxide, how are they classified
oxides are compounds made from one or more atoms of oxygen combined with one other element
oxides can be classified based on their acid-base characteristics
acidic and basic oxides; in terms of pH and metallic character
acidic and basic oxides have different properties and values of pH
the difference in their pH stems from whether they are bonded to a metal or a non-metal element
The metallic character of the element influences the acidic or basic behaviour of the molecule
acidic oxides
acidic oxides are formed when a non-metal element combines with oxygen
react with bases
when dissolved in water they produce an acidic solution with a low pH
e.g. CO2 and SO2
basic oxides
basic oxides are formed when a metal element combines with oxygen
react with acids to form a salt and water
when dissolved in water they produce a basic solution with a high pH
e.g. CuO and CaO
neutral oxides
some oxides do not react with either acids or bases and thus are said to be neutral
e.g. CO, NO and N2O
amphoteric oxides
curious group of oxides that can behave as both acidic and basic, depending on whether the other reactant is an acid or a base, in both cases salt and water are formed
e.g. Al2O3 and ZnO
(applies to their hydroxides as well)
aluminium oxide behaving as a base
Al2O3 + 6HCl gives 2AlCl3 + 3H2O
aluminium oxide behaving as an acid
Al2O3 + 2NaOH gives 2NaAlO2 + H2O
salt
a compound that is formed when the hydrogen atom in an acid is replaced by a metal
Naming salts involves 2 parts; the name of the metal and the end of the acid
eg. calcium + hydrochloric acid gives calcium chloride
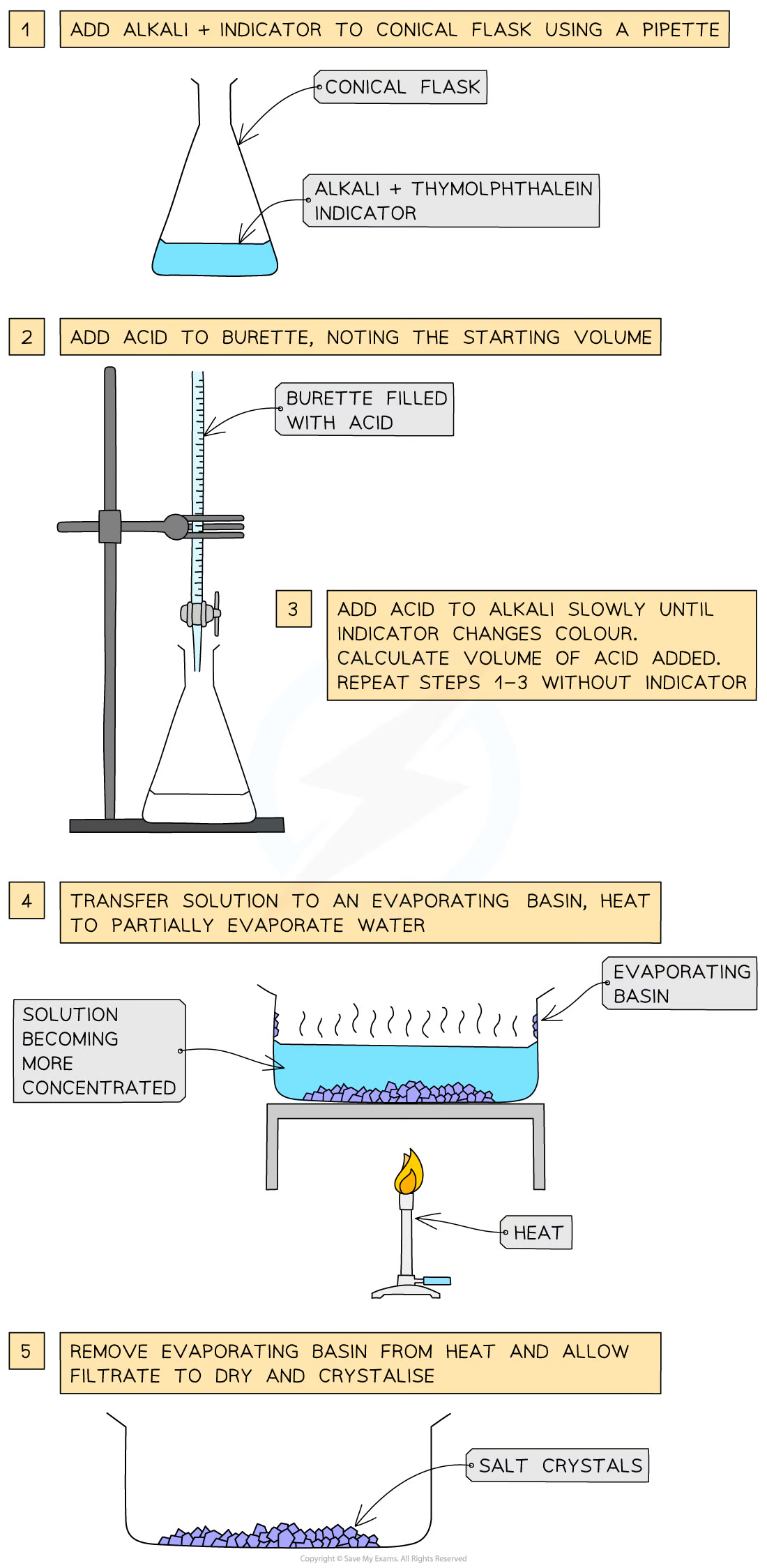
soluble salt preparation: dilute acid and alkali (soluble base) by titration
Use a pipette to measure the alkali into a conical flask and add a few drops of indicator (thymolphthalein or methyl orange)
Add the acid into the burette and note the starting volume
Add the acid very slowly from the burette to the conical flask until the indicator changes to the appropriate colour
Note and record the final volume of acid in the burette and calculate the volume of acid added (starting volume of acid - final volume of acid)
Add this same volume of acid into the same volume of alkali without the indicator
Heat the resulting solution in an evaporating basin to partially evaporate, leaving a saturated solution (crystals just forming on the sides of the basin or on a glass rod dipped in and then removed)
Leave to crystallise, decant excess solution and allow crystals to dry
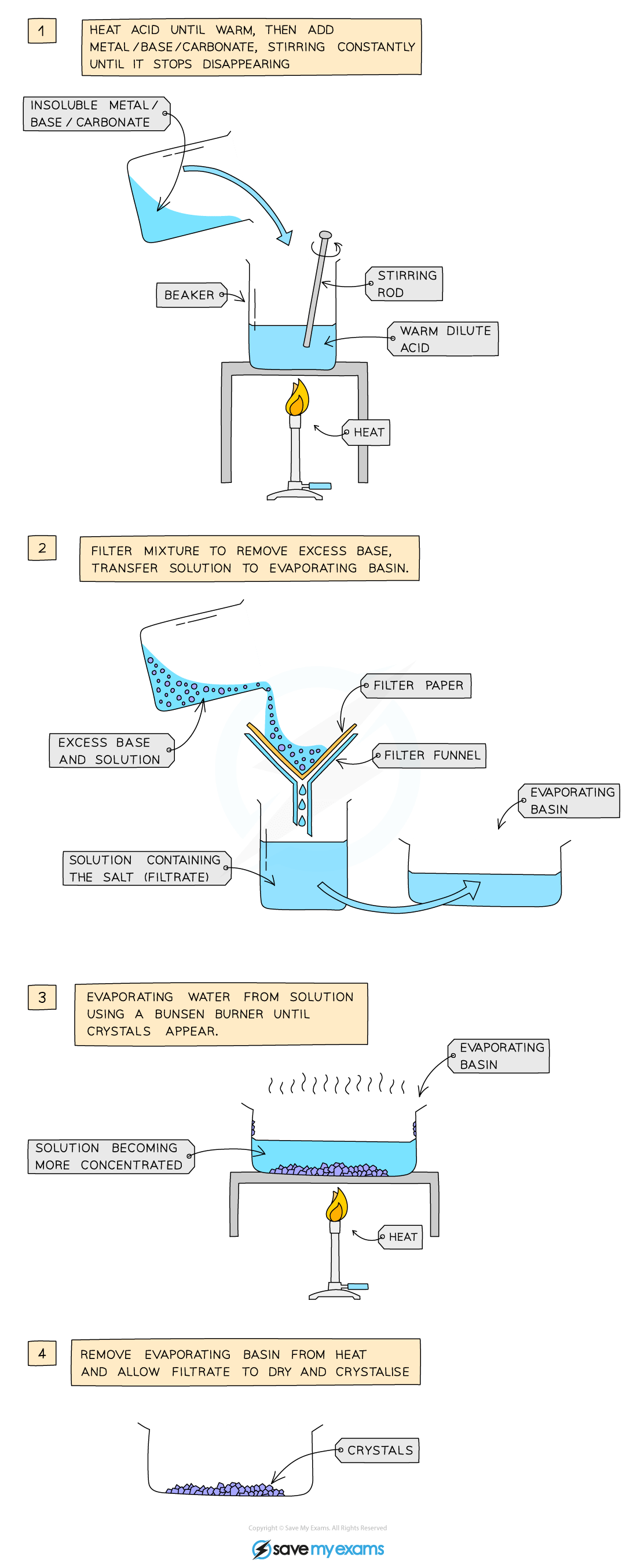
soluble salt preparation: acid to a solid metal, insoluble base or insoluble carbonate
Add dilute acid into a beaker and heat using a bunsen burner flame
Add the insoluble metal, base or carbonate, a little at a time, to the warm dilute acid and stir until the base is in excess (i.e. until the base stops disappearing and a suspension of the base forms in the acid)
Filter the mixture into an evaporating basin to remove the excess base
Heat the solution to evaporate water and to make the solution saturated. Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the filtrate in a warm place to dry and crystallize
Decant excess solution and allow crystals to dry or blot to dry with filter paper
example of soluble salt preparation: acid (dilute sulfuric acid) to insoluble base (copper(II) oxide)
copper(II) oxide + sulfuric acid gives copper(II) sulphate + water
CuO (s) + H2SO4 (aq) gives CuSO4 (aq) + H2O (l)
insoluble salt preparation: two soluble salts by precipitation
Dissolve soluble salts in water and mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash filtrate with distilled water to remove traces of other solutions
Leave in an oven to dry
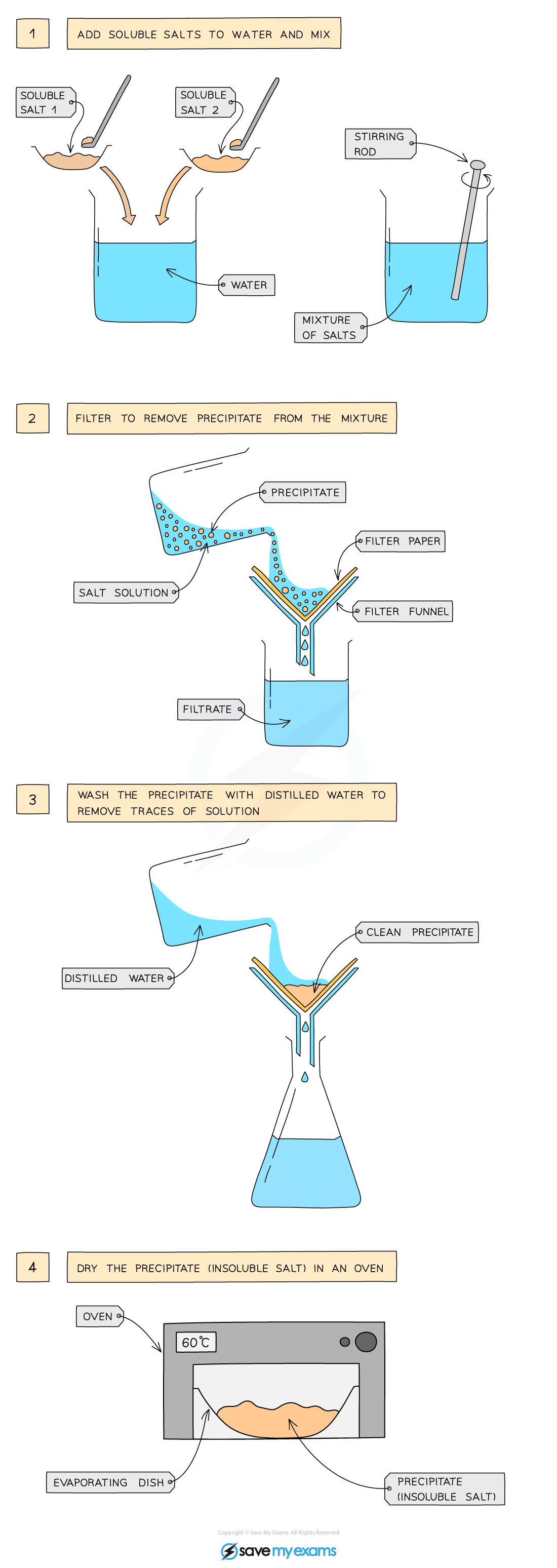
example of insoluble salt preparation: two soluble salts (lead(II) nitrate and potassium sulfate) by precipitation
lead(II) nitrate + potassium sulfate gives lead(II) sulfate + potassium nitrate
Pb(NO3)2 (aq) + K2SO4 (aq) gives PbSO4 (s) + 2KNO3 (aq)
solubility rules
sodium, potassium, and ammonium salts: all are soluble
nitrates: mostly soluble (except Ag, lead(II) Pb(NO3)2)
chlorides: mostly soluble (except Ba, Ca, lead(II) Pb(NO3)2)
carbonates: mostly insoluble (except Na, K, NH4)
hydroxides: mostly insoluble (except Na, K, NH4, Ca is partially soluble)
hydrated substance
a substance that is chemically combined with water
anhydrous substance
a substance containing no water
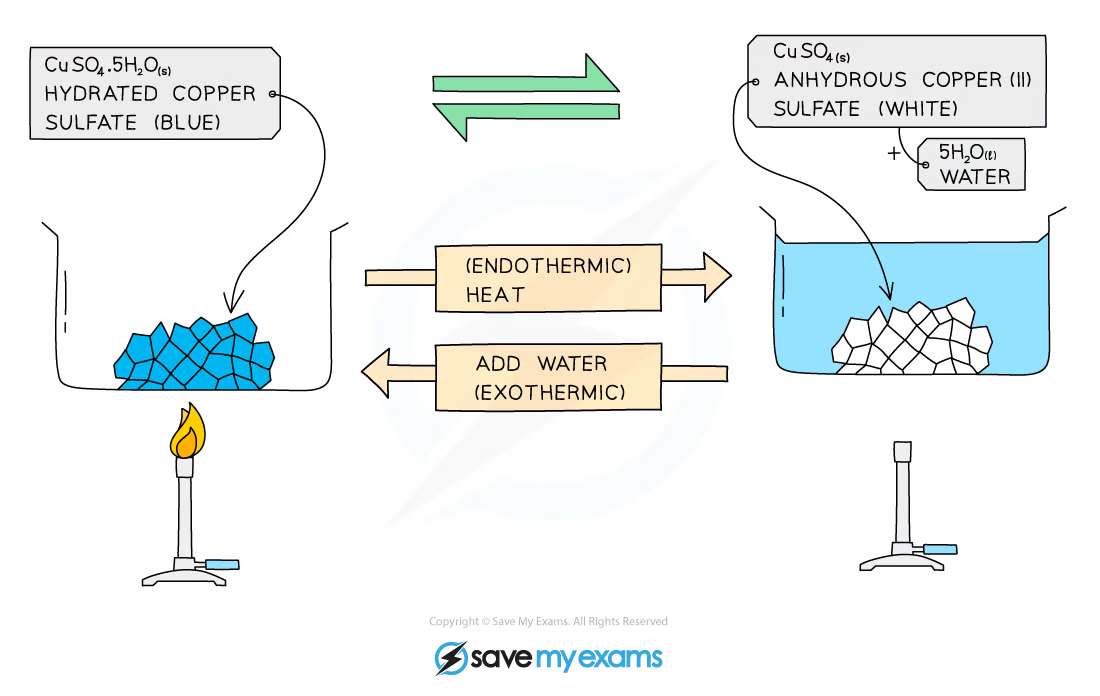
example of copper(II) sulfate becoming a hydrated and anhydrous salt
copper(II) sulfate crystallises forming the salt hydrated copper(II) sulfate, which is blue
when heated, the water from its structure is removed, forming anhydrous copper(II) sulfate, which is white
can be reversed by adding water to anhydrous copper(II) sulfate:
hydrated copper(II) sulfate reversibly gives anhydrous copper(II) sulfate + water
water of crystallisation
water molecules present in hydrated crystals
e.g. cobalt(II) chloride and copper(II) sulfate
anhydrous to hydrated salt:
CuSO4 + 5H2O gives CuSO4∙5H2O
CoCl2 + H2O gives CoCl2∙6H2O
hydrated to anhydrous salt (by heating):
CuSO4∙5H2O gives CuSO4 + 5H2O
CoCl2∙6H2O gives CoCl2 + H2O