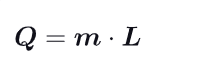3.1 Temperature & Heat
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
How to convert celcius to fahrenheit temperatures ?
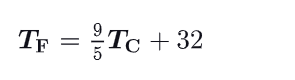
How to convert celcius to Kelvin temperatures ?
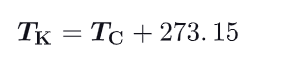
Mathematically, how does pressure & temperature relates ?
Linear relationship
When do gases reach zero pressure ?
Almost the same temperature : -273°C / absolute zero temperature 0K
What do gases experience at temperatures higher than absolute zero ?
Condensation
What is thermal expension ?
When a body is heated and its atoms and molecules displace forward to occupy a wider space.
How do solids experiment thermal expansion ? Give the formula associated with the change in length.
-each side expands along a line (elongation)
-cooling causes linear contraction
-The coefficient of proportionality alpha is called the “coefficient of linear expansion,” expressed in °C^-1
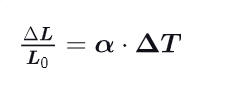
How do liquids experiment thermal expension ?
Since liquids assume the form of their containers, they would expand or contract volumetrically.
Beta = coefficient of volumetric expansion
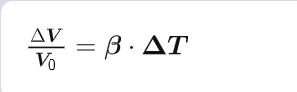
What is the relationship between alpha & beta ?
As alpha characterizes a unidirectional expansion & Beta a three-dimensional (3D) expansion
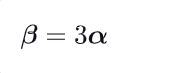
How is the amount of heat exchanged calculated ?
C (coefficient of prop.) = “heat capacity” in J/K
Q in J
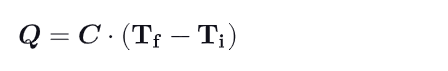
How is C calculated ?
Product of mass m of the system & specific heat
Specific heat is expressed in J/kg.K and is the amount of heat needed by a unit mass of a material to change its temperature by 1 degree.
What is Heat of Transformation L ?
Amount of heat absorbed or released during a phase change or chemical reaction. It is a measure of the energy required to transform a substance from one state to another, such as from solid to liquid or liquid to gas.
Give another formula for the exchange of Heat, using L :