(Part 2) MODULE 1. BONDING, STRUCTURE, AND REACTION MECHANISMS
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
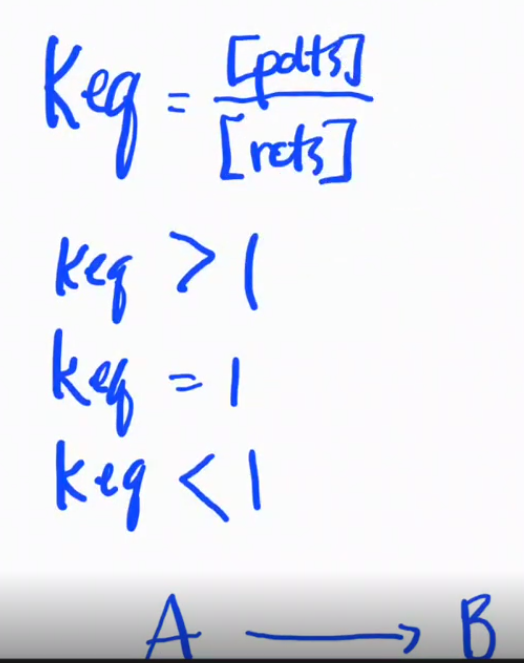
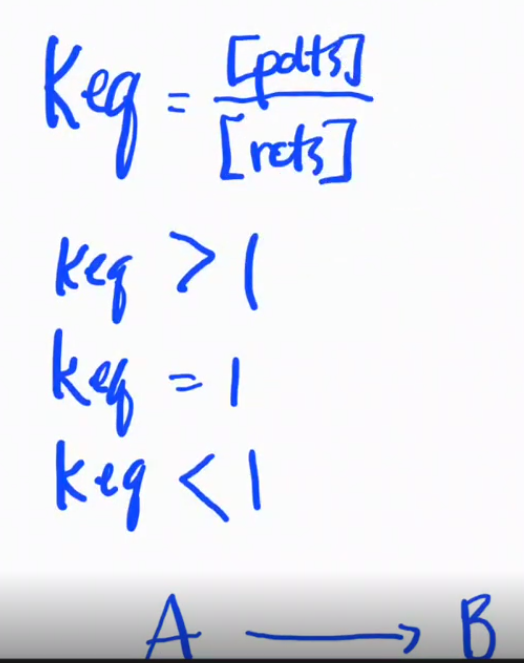

Gibs Free Energy change, ΔG
ΔG = —
energy is released to the surroundings
reaction is favored
exergonic
Gibs Free Energy change, ΔG
ΔG = +
energy is absorbed from the surroundings
reaction is not favored
endergonic

What are the two formulas for ΔG°
Enthalpy change, ΔH
- heat of reaction
- measure of change in total bonding energy during a reaction
ΔH = (-)
the bonds in the products are stronger (more stable) than the bonds in the reactant; heat is released (exothermic)
ΔH = (+)
the bonds in the products are weaker (less stable) than the bonds in the reactants; heat is absorbed (endothermic)




liberated; consumed
Energy is __________ when atoms combine to form molecules (i.e. bonds). When a molecule (i.e. bonds) breaks up into atoms, an equivalent amount is __________.
Exergonic reaction

What kind of reaction is this?
exergonic
A reaction that proceeds with a negative energy change (releases energy to its surroundings) is said to be _____________.
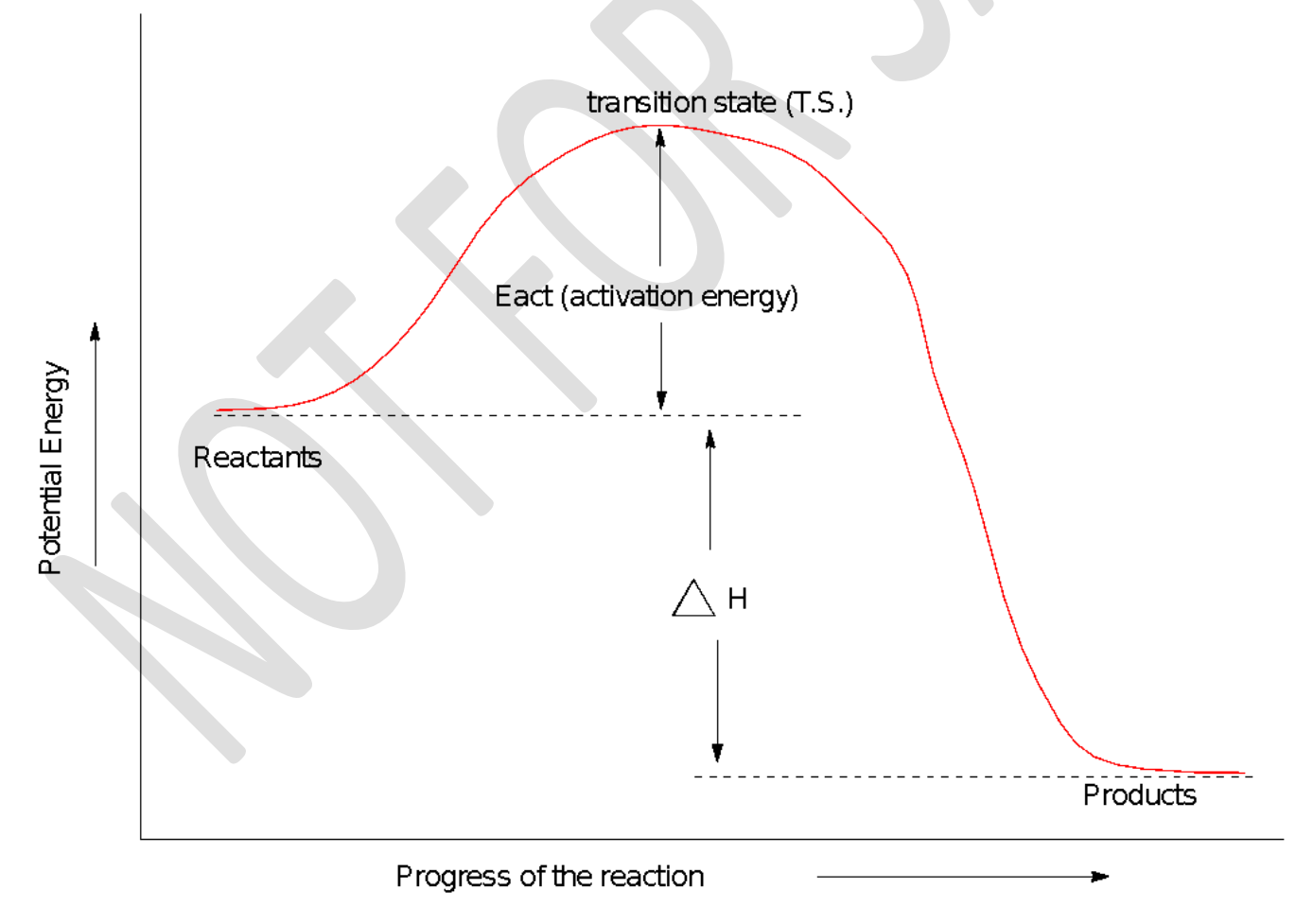
transition state (T.S); activation energy
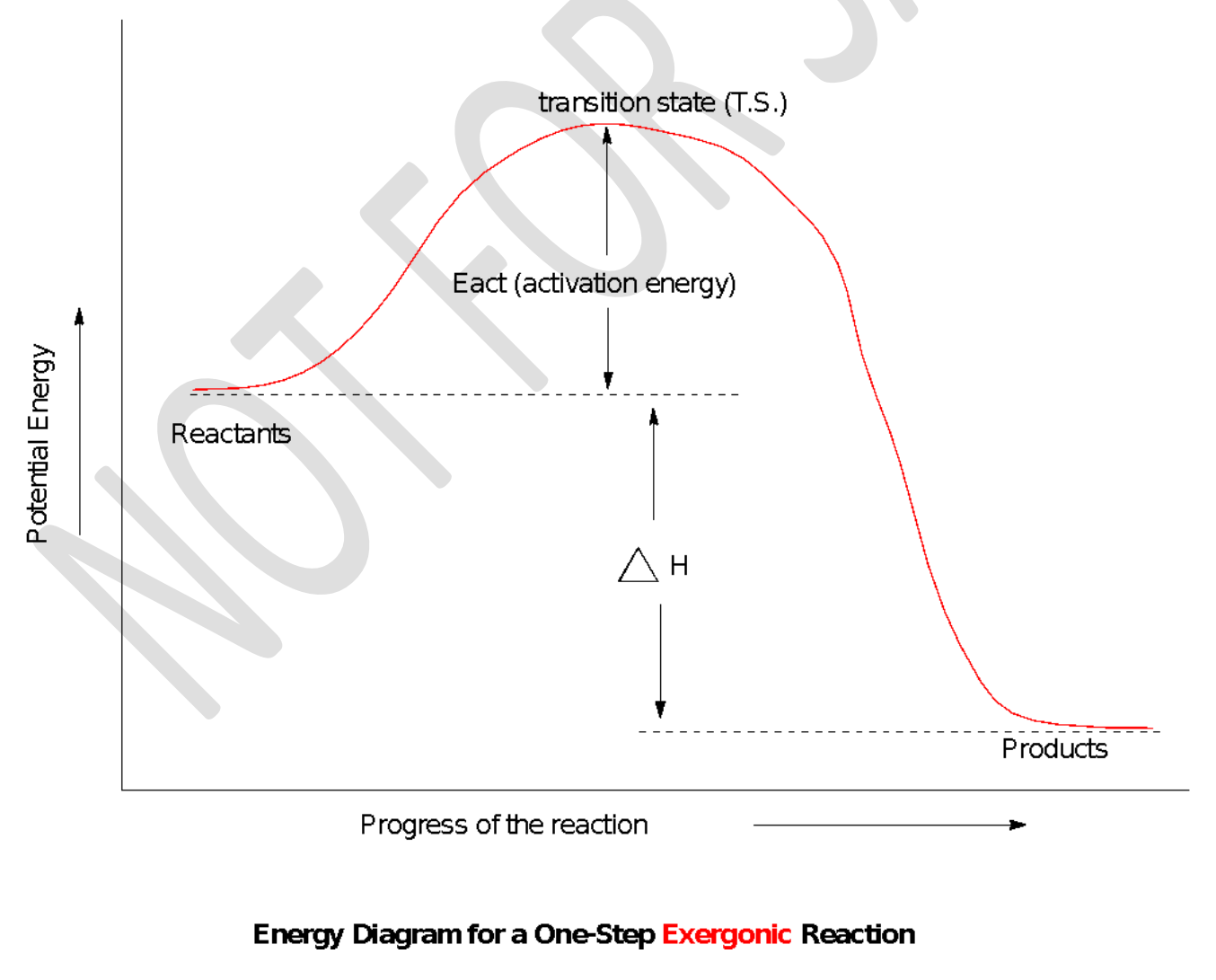
The top of the energy curve corresponds to the _____________ for the reaction. The ____________ for the reaction is the difference in energy between the reactants and the transition state.
endergonic
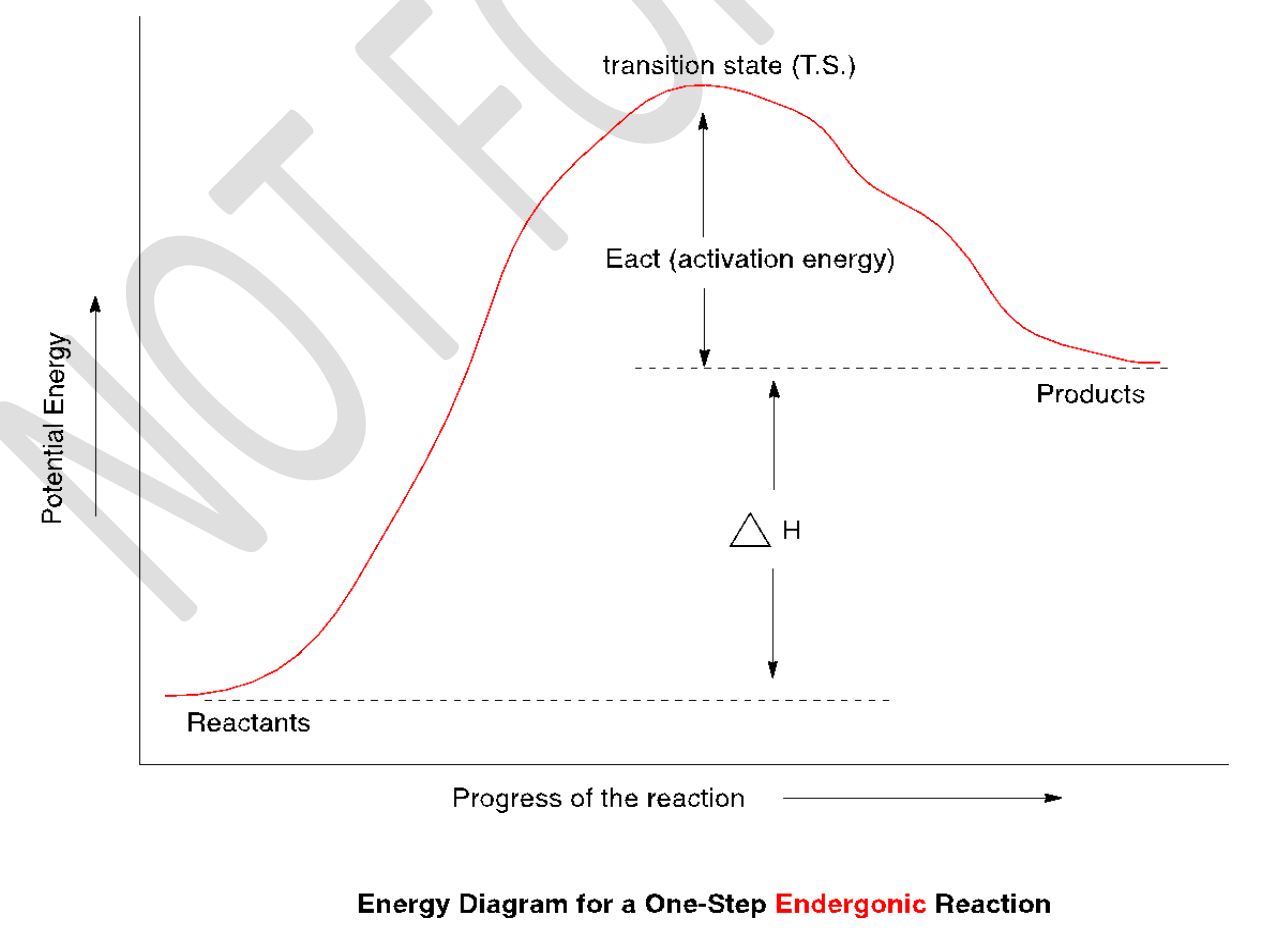
That is, if the products have greater energy than reactants, the energy of activation will be even higher. The reaction that proceeds with a positive energy change (absorbs energy from its surroundings) is said to be ____________.
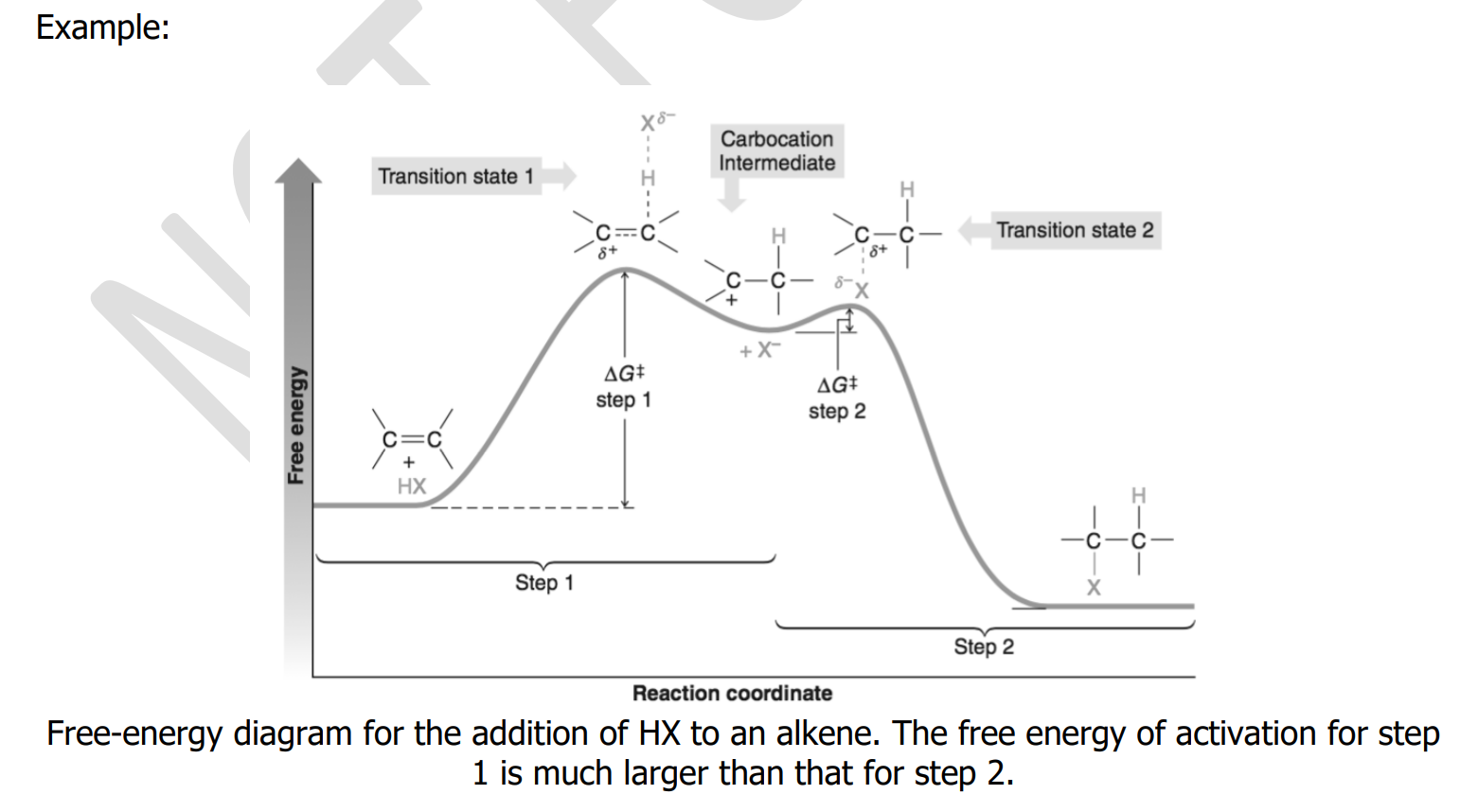
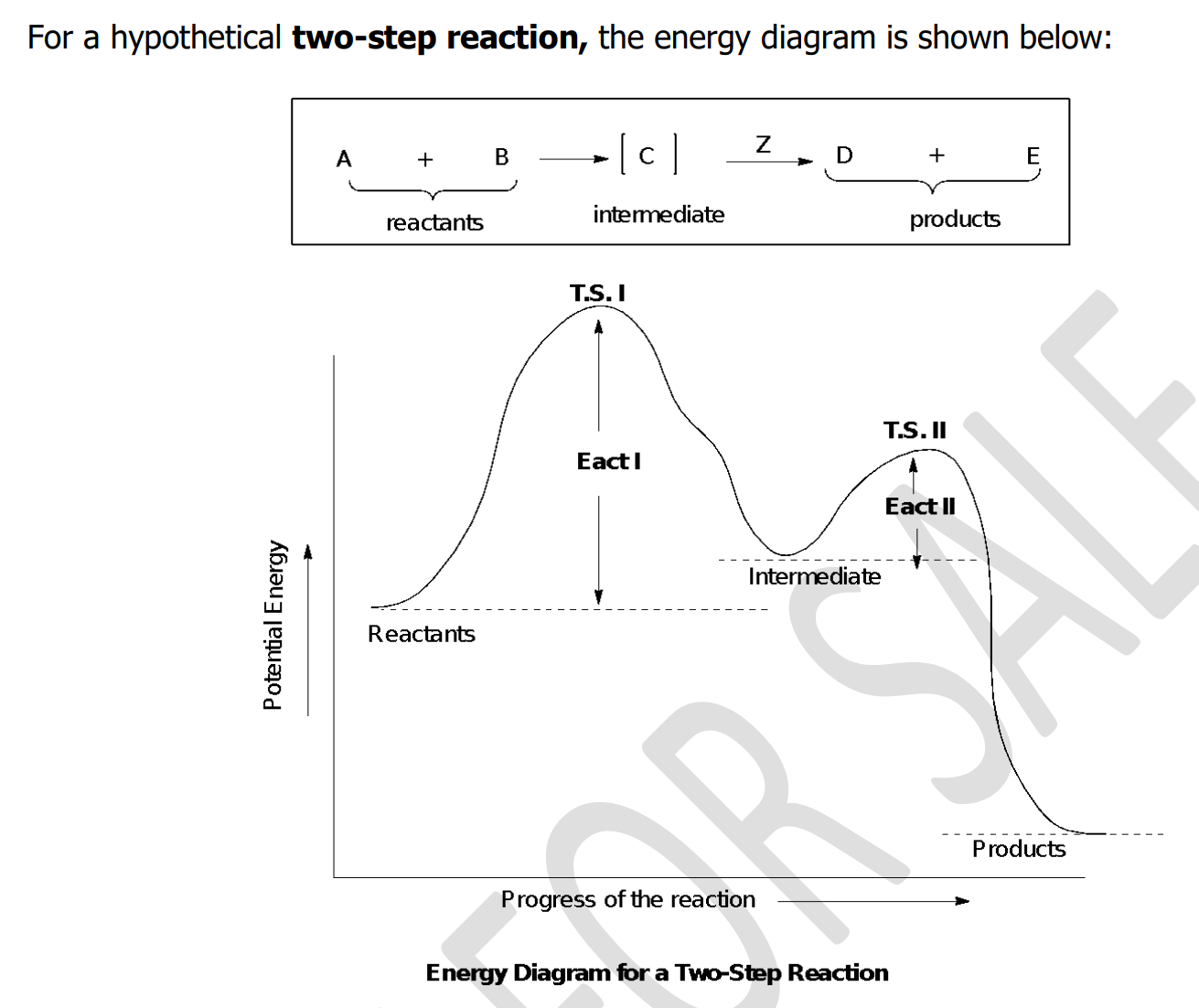
rate-determining; endergonic; exergonic

The important step—because it is the _______________ step—is step 1. This step is highly __________ and has a high free energy of activation. Consequently, it takes place slowly. In step 2 the highly reactive carbocation stabilizes itself by combining with a halide ion. This __________ step has a very low free energy of activation and takes place very rapidly.
Intermediate
- a species that is formed during the course of a multistep reaction but is not the final product
Intermediate
- more stable than transition states but may or may not be stable enough to be isolated
Types of Bond Cleavage
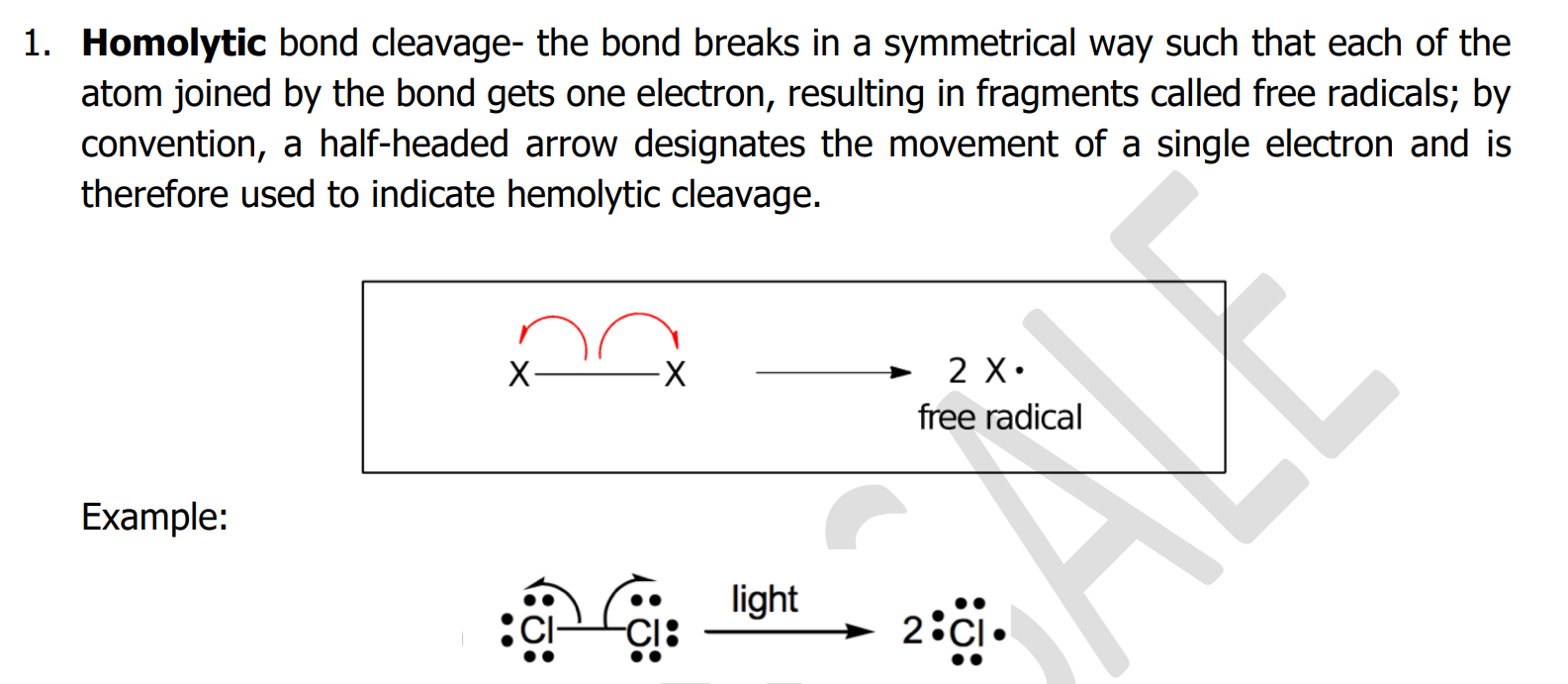
Homolytic bond cleavage
Types of Bond Cleavage
- the bond breaks in a symmetrical way such that each of the atom joined by the bond gets one electron, resulting in fragments called free radicals;
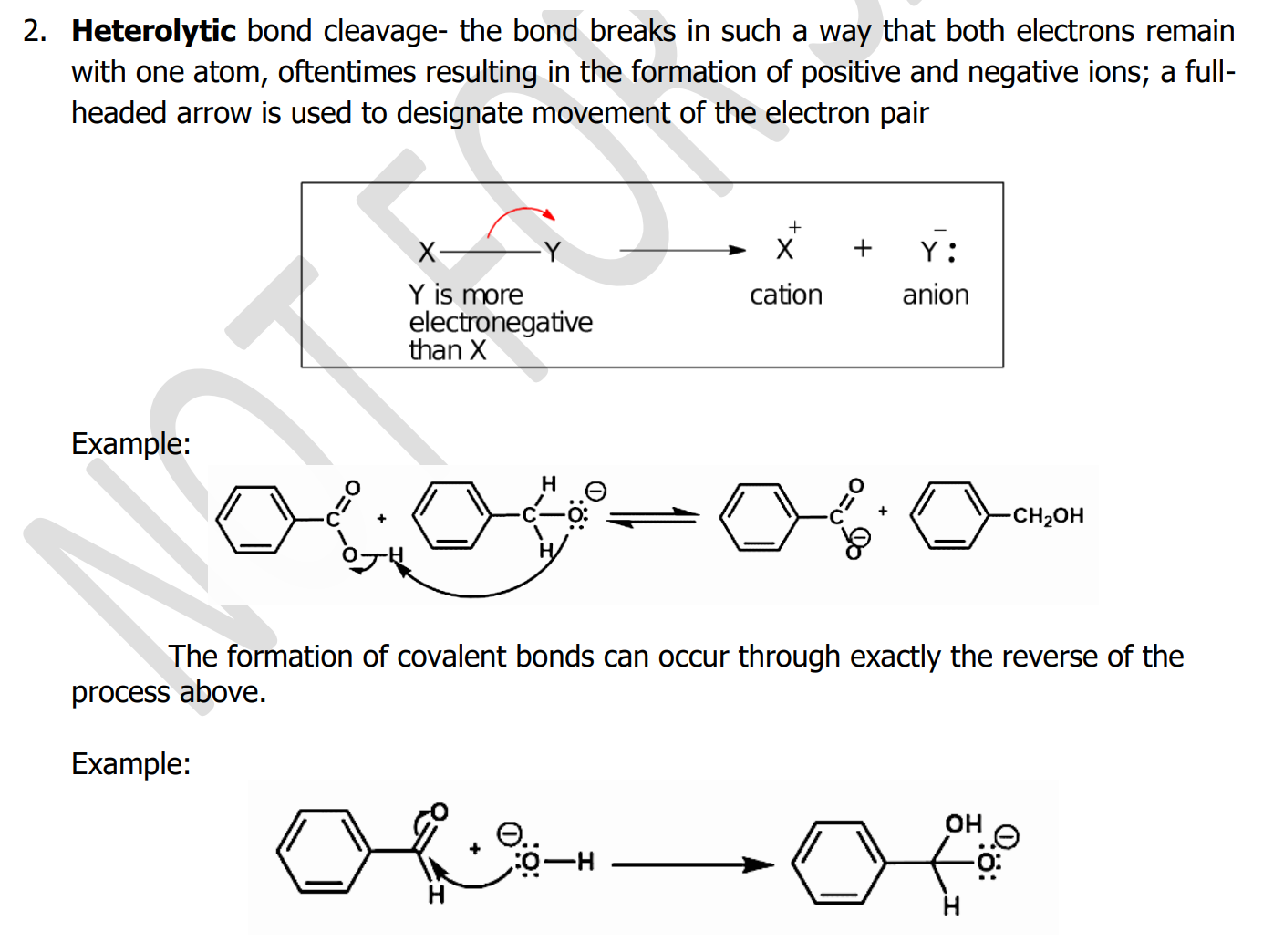
Heterolytic bond cleavage
Types of Bond Cleavage
- the bond breaks in such a way that both electrons remain with one atom, oftentimes resulting in the formation of positive and negative ions;
Electrophilic (electrophile)
Types of Reagents
electron-loving; usually Lewis acids
Electrophilic (electrophile)
Types of Reagents
-species that are electron-deficient, and are capable of forming new bonds by accepting a pair of electrons; they attack positions of high electron density
Nucleophilic (nucleophile)
Types of Reagents
nucleus-loving; usually Lewis bases
Nucleophilic (nucleophile)
Types of Reagents
-species that have pairs of electrons that can be used in bond formation; they attack positions with positive charge or low electron density
Radical-like or ‘free radical‘
Types of Reagents
- species that have an odd number of electrons; they are very reactive, and their tendency is to pair up the single electron
a chemical species that contains an odd number of valence electrons and thus has an orbital with only one electron
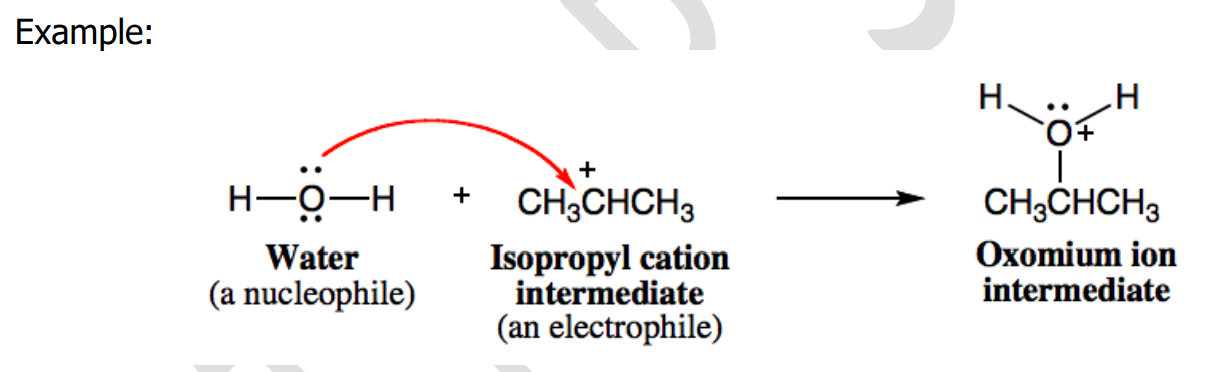
Heterolytic
Types of Reagents
___________ bond cleavage occurs when an electrophilic or nucleophilic reagent attacks a substrate. The reaction is called an electrophilic or nucleophilic reaction.
Homolytic
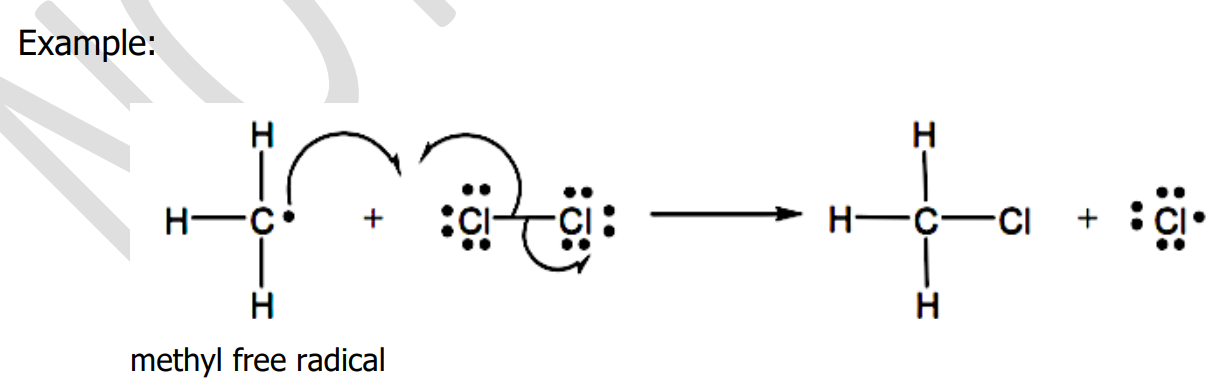
_____________ bond cleavage occurs when a radical-like reagent attacks a substrate. The reaction is called free-radical reaction.