CELL BIO EXAM 3
1/166
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
167 Terms
extracellular matrix
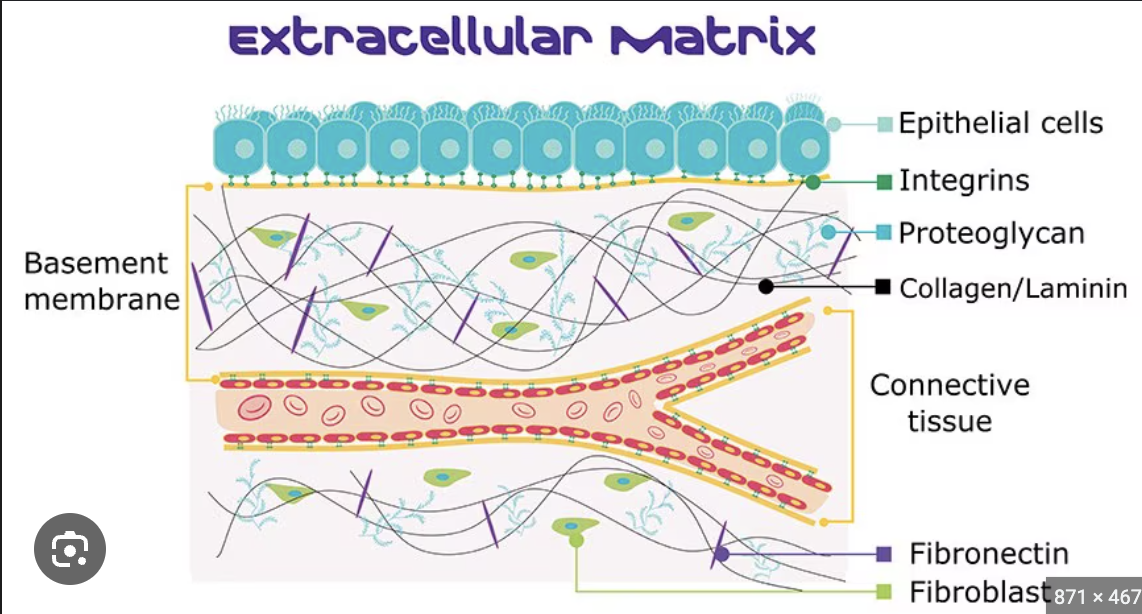
extra = “outside”, matrix =”beyond”. a unique, tissue-specific environment that is made of structural and functional biomacromolecules. (FIBERS- sheet-forming+ fibrillar collagen), organizers (e.g., fibronectin, laminin, nidogen/entactin), and proteoglycans (e.g., perlecan) in a 3D structure.
KNOW WHERE everything is located on an endothelial cell
Major components of the ECM?
Proteoglycans (including perlecan) organizers (including nidogen/entactin, laminin, and fibronectin), fibers (including sheet-forming (type IV, and fibrillar collagens (type 1,2,3,5).
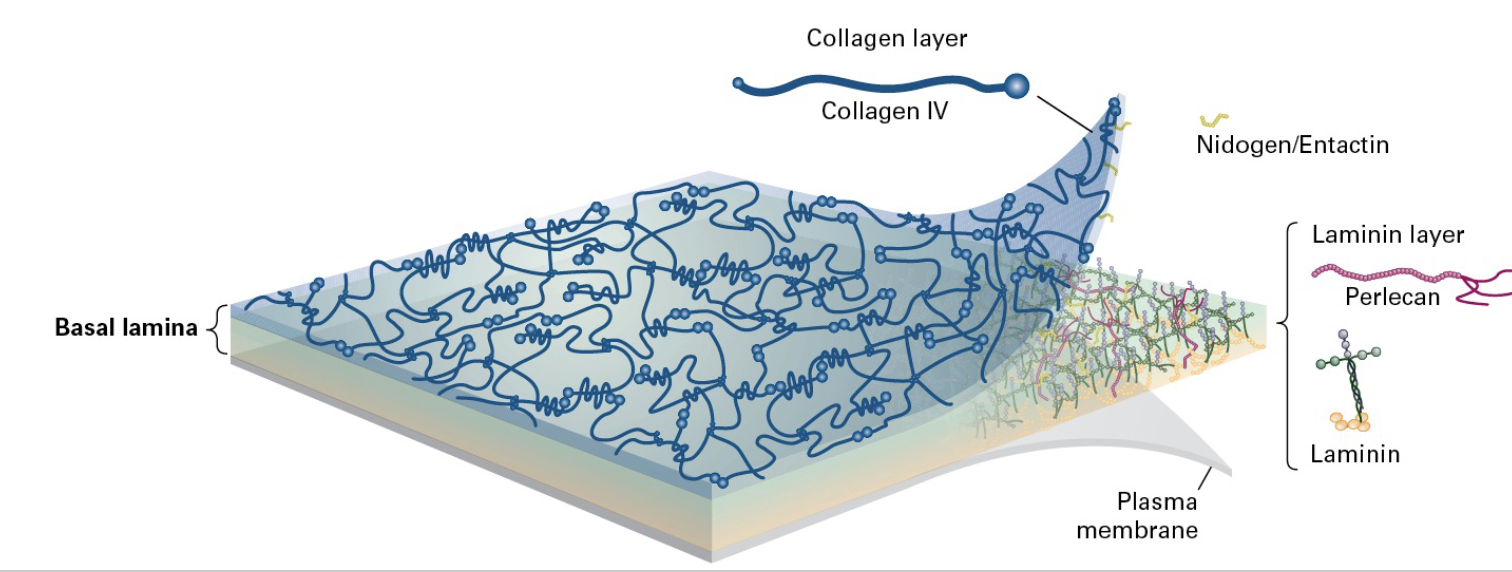
what is the basement membrane (AKA basal lamina?)
The basal lamina is a 2 ply structure; laminin makes up one of them and collagen the other and pelicans and nidogen cross link everything together.
Type 4 collagen and laminin make up the sheets of the two-ply structure. (composed of materials excreted by epithelial cells such as laminin, glycoproteins, and type IV collagen)
Sheets of epithelia sit on basal lamina
• Mat-like structure
• Composed Mainly of Type IV Collagen, Laminin, and Nidogen
• Provides connection sites for Integrins
What are Proteoglycans?
*Proteoglycans play a role in ECM adhesion. Covalently linked to specialized polysaccharide side chains (GAGs). glycosaminoglycans. Proteoglycan cores are synthesized in the ER.
These are essential components of the ECM, playing roles in cell signaling, hydration, and providing structural support.
Located in the basement membrane and interacts with other proteins like laminin and type 4 collagen.
What are GAGs (including the four types!)
Four classes of GAGs are characterized by disaccharide repeats w/modifications (e.g. sulfate grp).
(1) hyaluronan (2) Chondroiton (or dermatan) sulfate (3) Heparin/Heparan sulfate (4) Keratan sulfate
GAGs (besides Hyaluronan): attach to a core protein to form proteoglycans and typically have sulfate modifications.
Hyaluronan: is a non-sulfated GAG that does not attach to a core protein and exists independently in the ECM.
GAGs; GAG chains are assembled and attached in the Golgi. Some GAGs are o-linked (attached by OH in serine or threonine R groups). Other GAGs are N-linked (attached by the R group of asparagine residues). *present in every mammalian tissue.
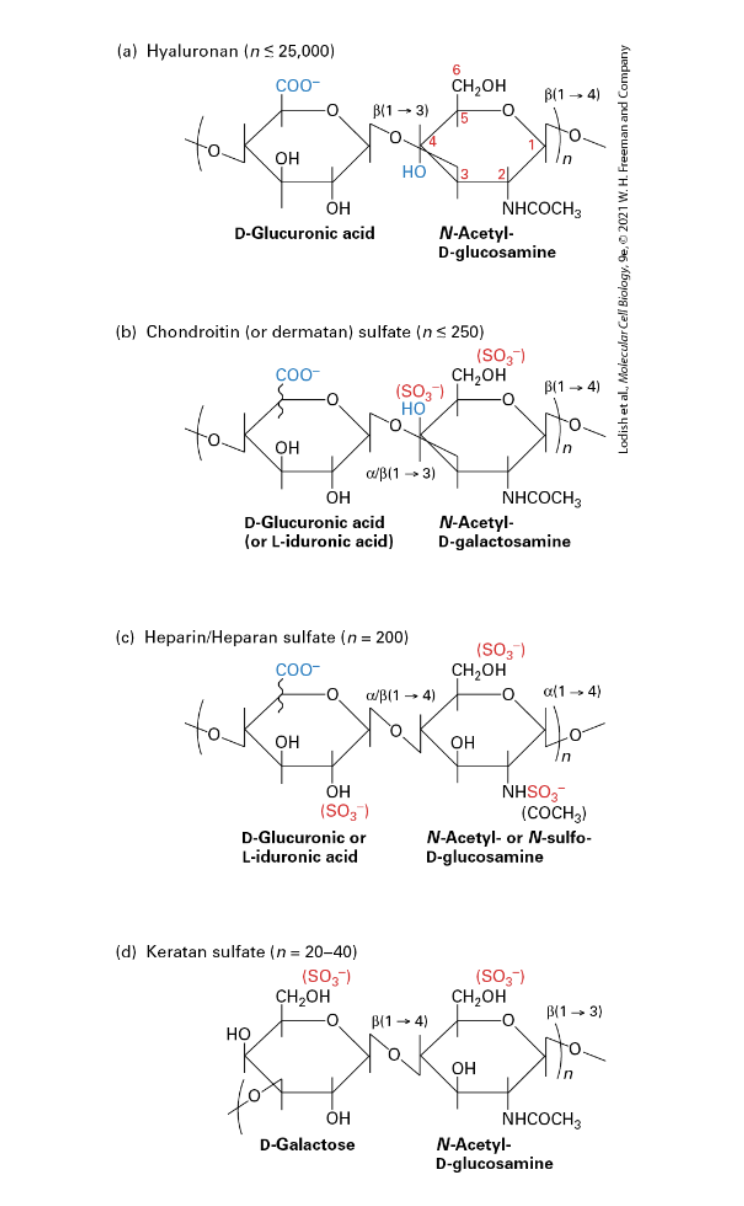
What is HYALURONAN/HA?
(Hyaluronic Acid, HA); aka hyaluronate, non-sulfated GAG. A major component of ECM. Made by a membrane-bound enzyme HA-synthase. Secreted directly into ECM (other GAGs secreted into ECM after associating w/a proteoglycan).
Length from a few disaccharide repeats to 25,000.
Acts like a hydrated sphere/gel. Resists compression forces. A major component of cartilage.
What is perlecan?
Perlecan: This specific proteoglycan is found in basement membranes and is important for maintaining tissue architecture. It can interact with other ECM proteins (such as nidogen and laminin), cells, and growth factors.
ROLES of ECM:
(1) anchoring + surrounding cells to maintain shape and define tissue boundaries. (2) Determining the biomechanical properties (stiffness/elasticity, porosity, shape) of the extracellular environment (3) Controlling cellular polarity, survival, proliferation, differentiation, responses to the environment and to disease (4) Inhibiting or facilitating cell migration (e.g., serving as either a barrier to movement or as a “track” along which cells can move) (5) Binding to and acting as a reservoir of growth factors; in some cases, the ECM (a) helps generate an extracellular concentration gradient of GF, (b) serves as a co-receptor for the GF, or (c) aids in proper binding of the GF to its receptor. (6) Activating cell surface signaling receptors.
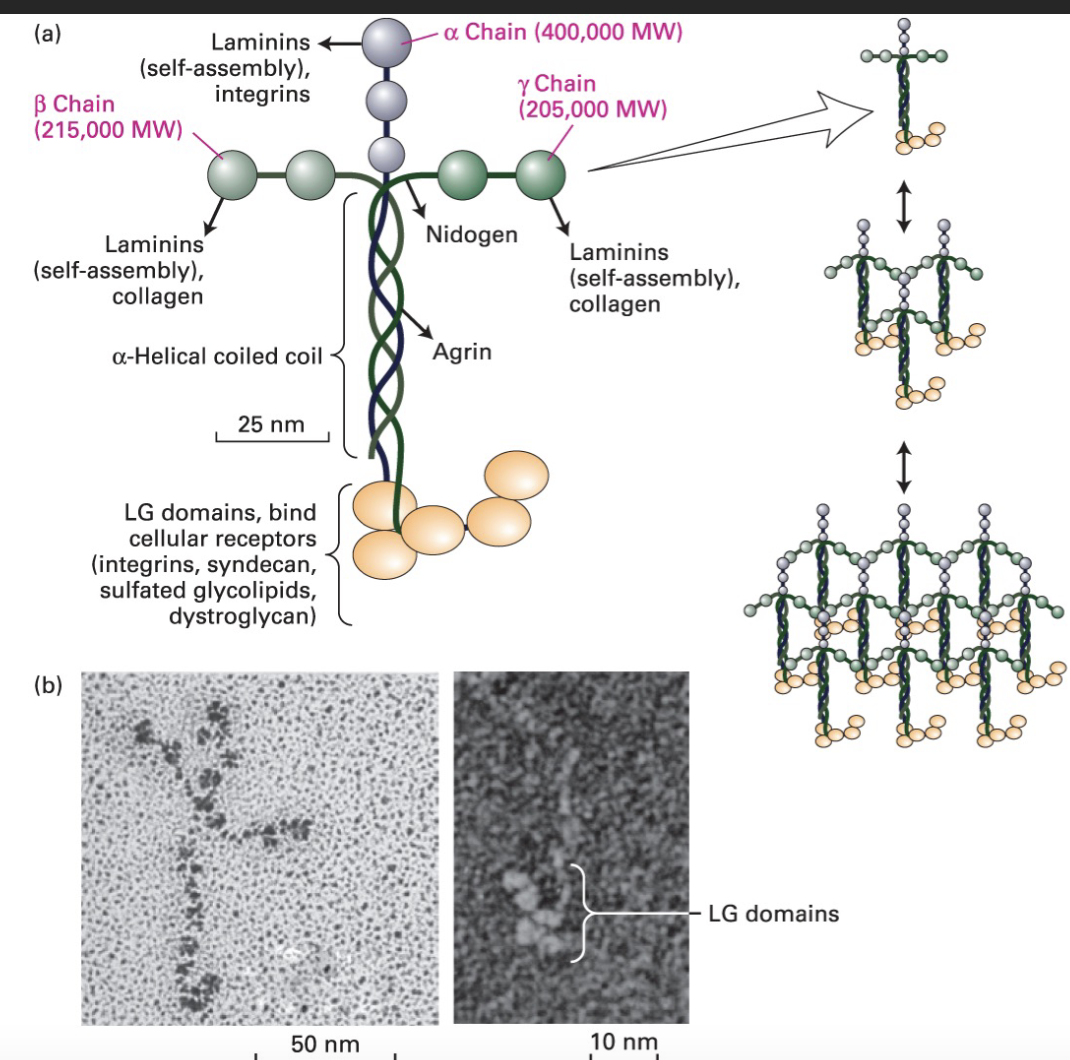
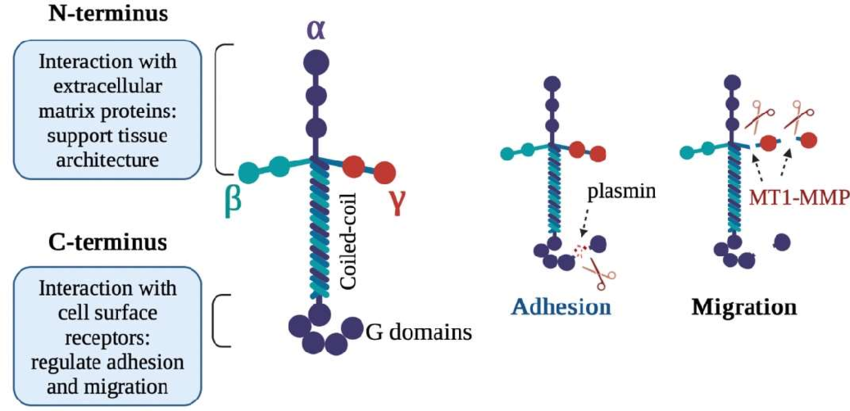
What are Laminins?
Laminins are multi-adhesive glycoproteins found in the basal lamina, structured as cross-like trimers composed of one heavy α chain and two light β and γ chains. They self-assemble into large structures through their globular domains, with C-terminal domains that bind cell receptors, including integrins like α6β4.
In the ECM laminins provide structural support tensile strength, and cell adhesion. They also act as ligands for specific integrins, facilitating cell-matrix interactions
Laminin acts as a ligand for the α6β4 integrin.
What is fibronectin?
Fibronectin is an adhesive glycoprotein that is primarily involved in cell–adhesive interactions. (promotes cell adhesion and migration).
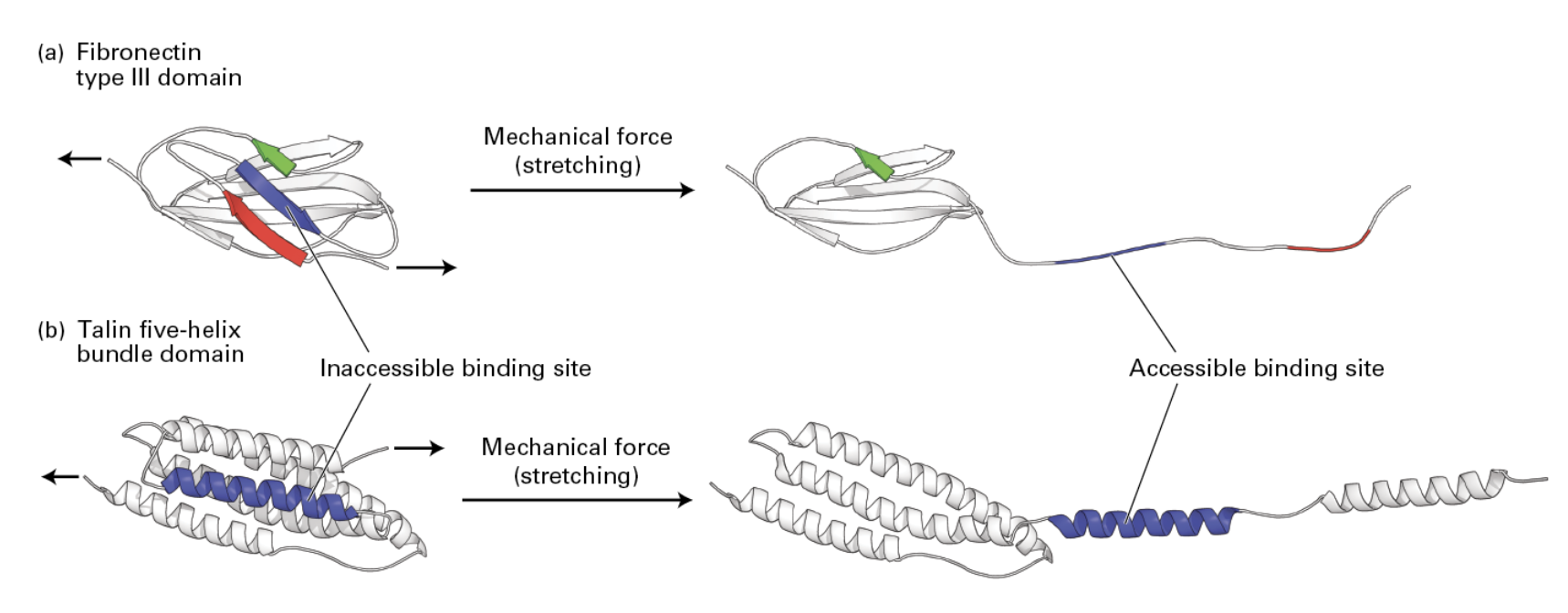
Fibronectin stretching in the ECM from cells nearby can cue the recruitment of further proteins for reinforcement by revealing binding sites.
It’s an abundant component of the ECM found in all
vertebrates. Dimers of two similar polypeptides linked at the C-terminus by disulfide bonds (~60-70 nm long, 2-3 nm thick)
Helps attach cells to ECM by binding other ECM components, including fibrillar collagen, proteoglycans, and adhesion receptors
• Domains are Type I, II, or III (according the repeats)
• Arg-Gly-Asp (RGD) domain binds Integrin.
What is nidogen/entactin?
Nidogen/entactin is believed to play a key role in the formation and organization of basement membranes since it binds separately to laminin, collagen IV, and heparan sulfate proteoglycan and promotes the assembly of complexes of these components.
What is Collagen?
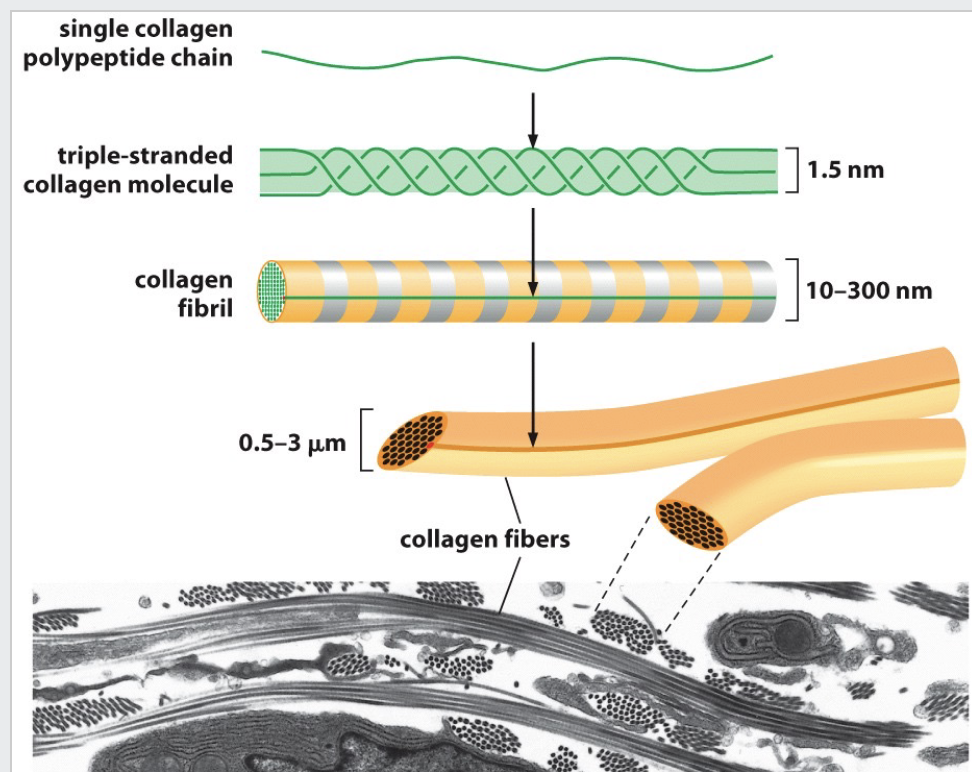
Collagens are trimeric molecules that often bundle to form fibers. Microfibrils (300 nm) associate to fibrils which bundle to form long fibers.
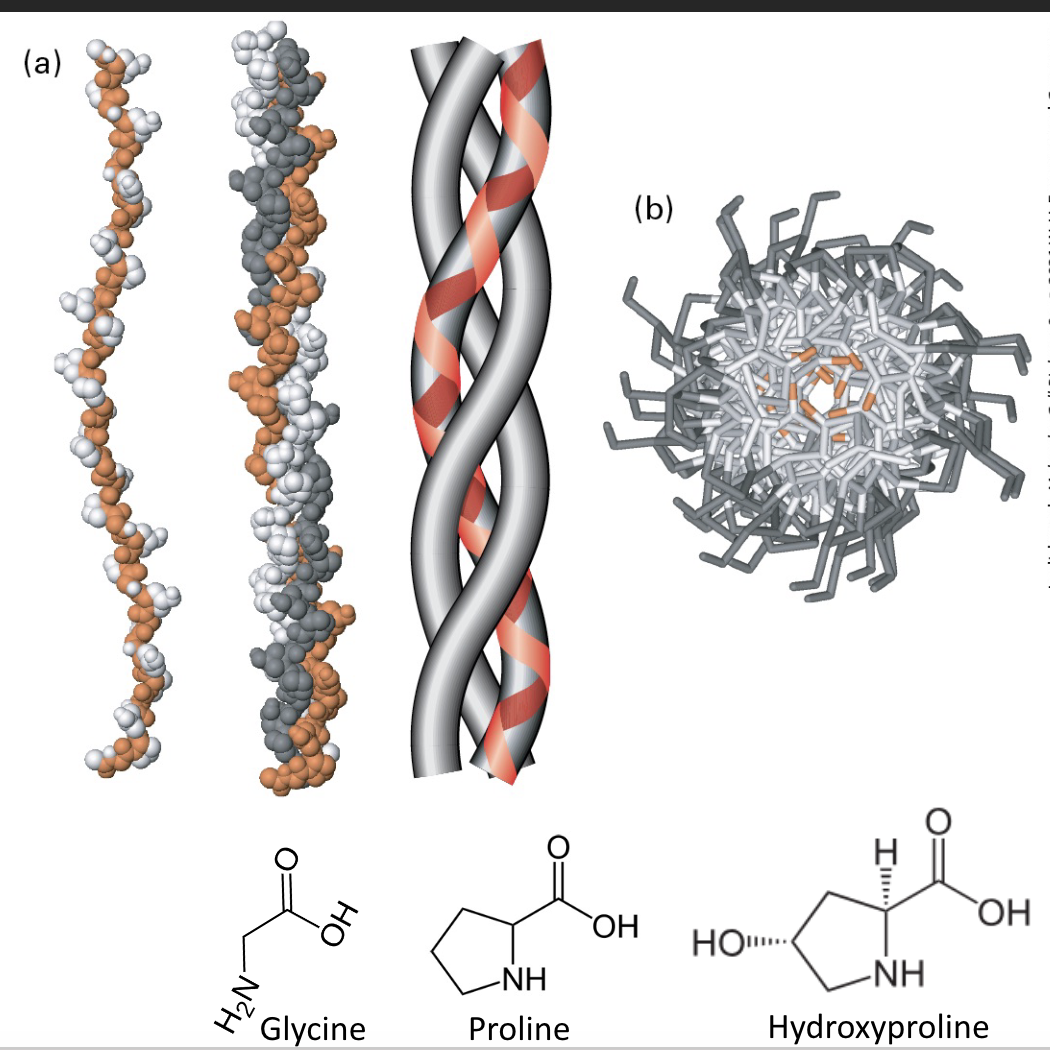
Collagen is a triple-helical bundle formed from left-handed helical proteins. The G (orange) stays in the center of the triple helix.
• Characteristic Glycine-X-Y (G-X-Y) repeats. X is often proline. Y is often hydroxyproline.
25% total protein in animals.
• Collagen fibers = superstructure
• Built by: Fibroblasts - skin, Osteoblasts - bone
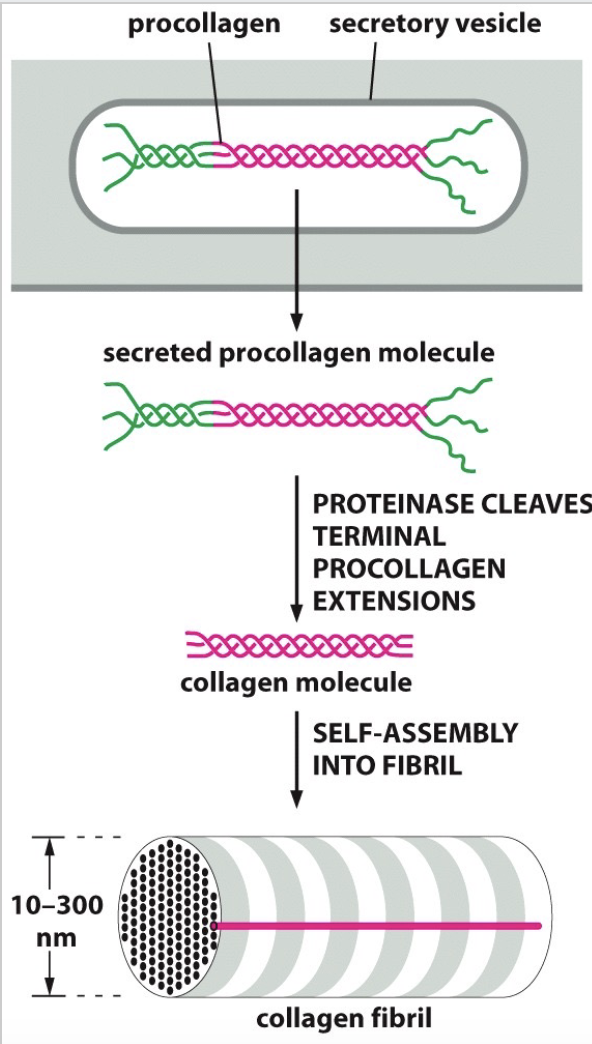
What happens with improper collagen assembly? (ALSO what is the process of it’s assembly?)
TOO stretchy skin.
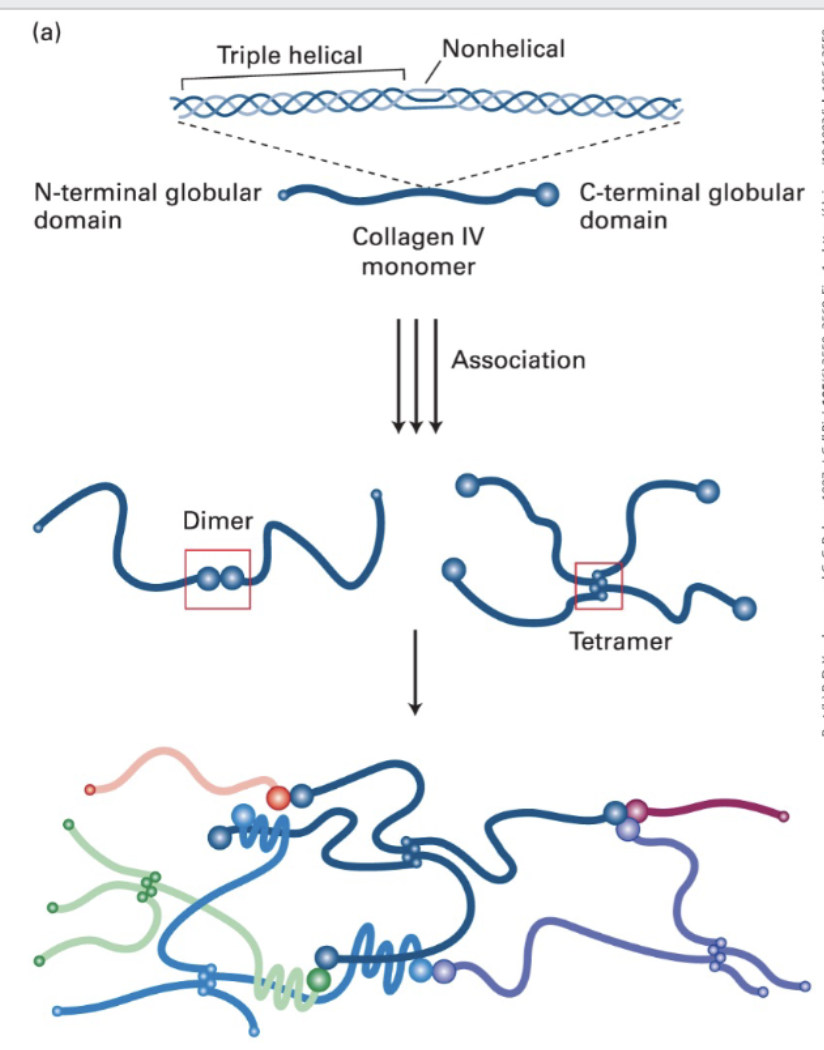
Sheet-forming and anchoring (collagen) fibers (Type IV (4))
molecular composition; [a1(IV)]2[a2(IV)] ; two-dimensional network. found in all basal laminae.
Type IV form 400 nm long triple helical structures with 24 non-helical interruptions (flexibility)
• They become covalently cross-linked to form a network that associates with the laminin lattice to form the basis of the basal lamina.
• Sheet-forming and anchoring collagens form 2-D networks in the basal lamina (e.g., Type IV)
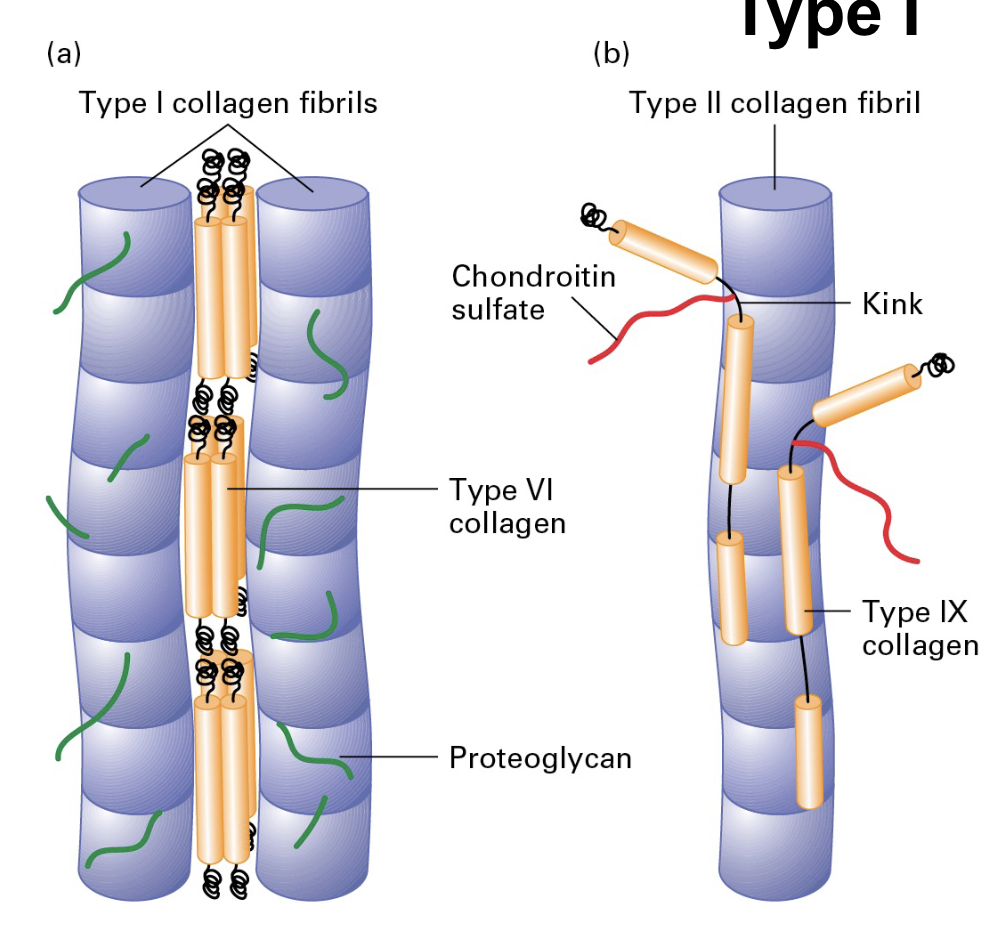
Fibrillar collagens (types 1,2,3)
ONLY need to know type 1;
• 80-90% of the human body’s collagen is fibrillar (Type I, II, III) mostly in connective tissue
• Type I has great tensile strength (stronger than steel, gram-for-gram)
• Type I is used to reinforce bone (30% protein, 70% mineral).
What are fibril-associated collagens?
• Fibril-associated collagens link fibrillar collagens to one another or to the ECM (type
Why do cells need cell adhesions?
Multicellularity requires connections between the cells. Distinct adhesions have distinct purposes/roles to play. Without adhesions multicellularity is hard to achieve.
What are adhesion receptors?
Cell Surface molecules engaged in adhesion are called adhesion receptors; Cell-Cell Adhesion, Cell-Matrix Adhesion
Aka, Cell Adhesion Molecules (CAMs); Important for communication. No communication = bad for the system
CAM families: Cadherins, Immuglobulin (Ig) superfamily, integrins, and sugar-binding proteins called Lectins.
Types of Interactions between Adherence molecules;
Homophilic and heterophilic interactions;
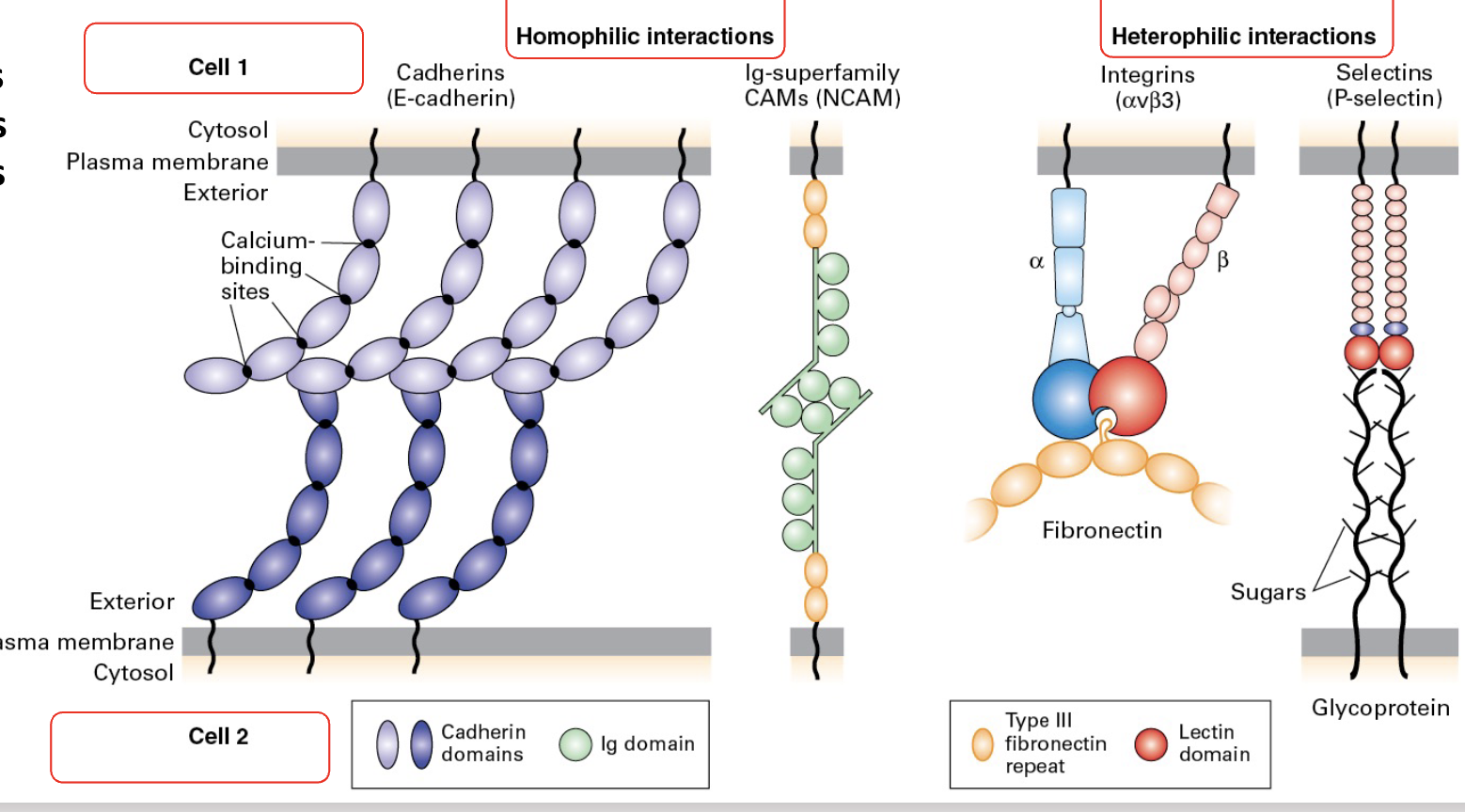
What are Cadherins/Cadherin Zippers?
(A type of CAM) Cadherin molecules interact with each other in the same cell (cis) or with adjacent cells (trans) to create a zipper.
• The ability to mix-and-match makes them a versatile family of proteins.
Individual interactions = weak, en masse = strong
Adherens junctions, through cadherin zippers, provide cell-cell connections contributing to tissue cohesion and stability while also interacting with the cytoskeleton for structural support.
What are immuglobulin superfamilies?
Many IgSF members are involved in cell-cell adhesion. They participate in forming adherens junctions, immune synapses, and other cellular interactions. (is a type of CAM)
What are integrins?
Integrins are a large family of alpha/beta heterodimeric cell surface proteins. Mediate both cell-cell and cell-matrix interactions
• Exhibit differing conformational states that alter affinity for targets. • Useful in dynamic interactions (e.g, immunity)
• a6b4 is the main laminin binding one in epithelium.
Integrins Connect ECM to the Cytoskeleton; The cytosolic domains of the integral proteins can participate in signaling and/or connect to the cell cytoskeleton.
Recruitment of talin to modified phospholipids allows association. Association in turn causes activation, dimerization, complex building, integrin activation, recruitment and force.
Integrins themselves do not bind directly to the extracellular matrix (ECM) without the help of other proteins like talin.
What is the process of Integrin activation?
Inactive Integrins: Integrins exist in an inactive form in the cell membrane, unable to effectively bind to ECM proteins.
Talin Binding: Talin binds to the cytoplasmic domain of integrins, which induces a conformational change that activates the integrin. This activation allows integrins to better interact with ECM components like fibronectin or laminin.
Formation of Focal Adhesions: Once activated, integrins can bind to ECM proteins. Talin further links the integrins to the actin cytoskeleton, forming structures called focal adhesions. These adhesions are crucial for transmitting forces and signals between the cell and the ECM.
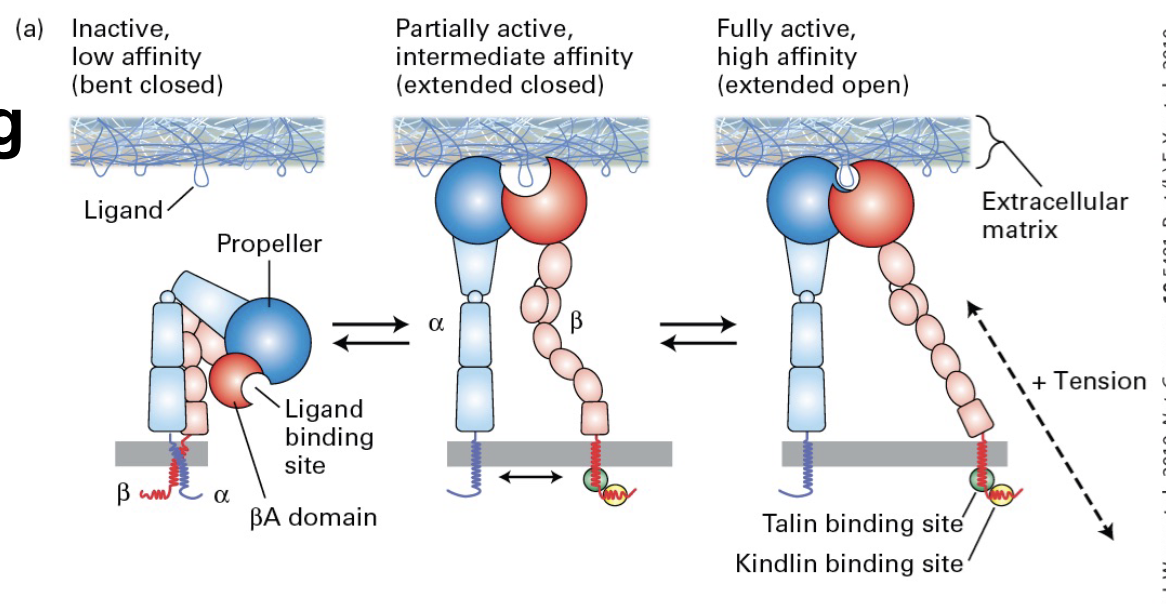
Different Affinities of Integrins
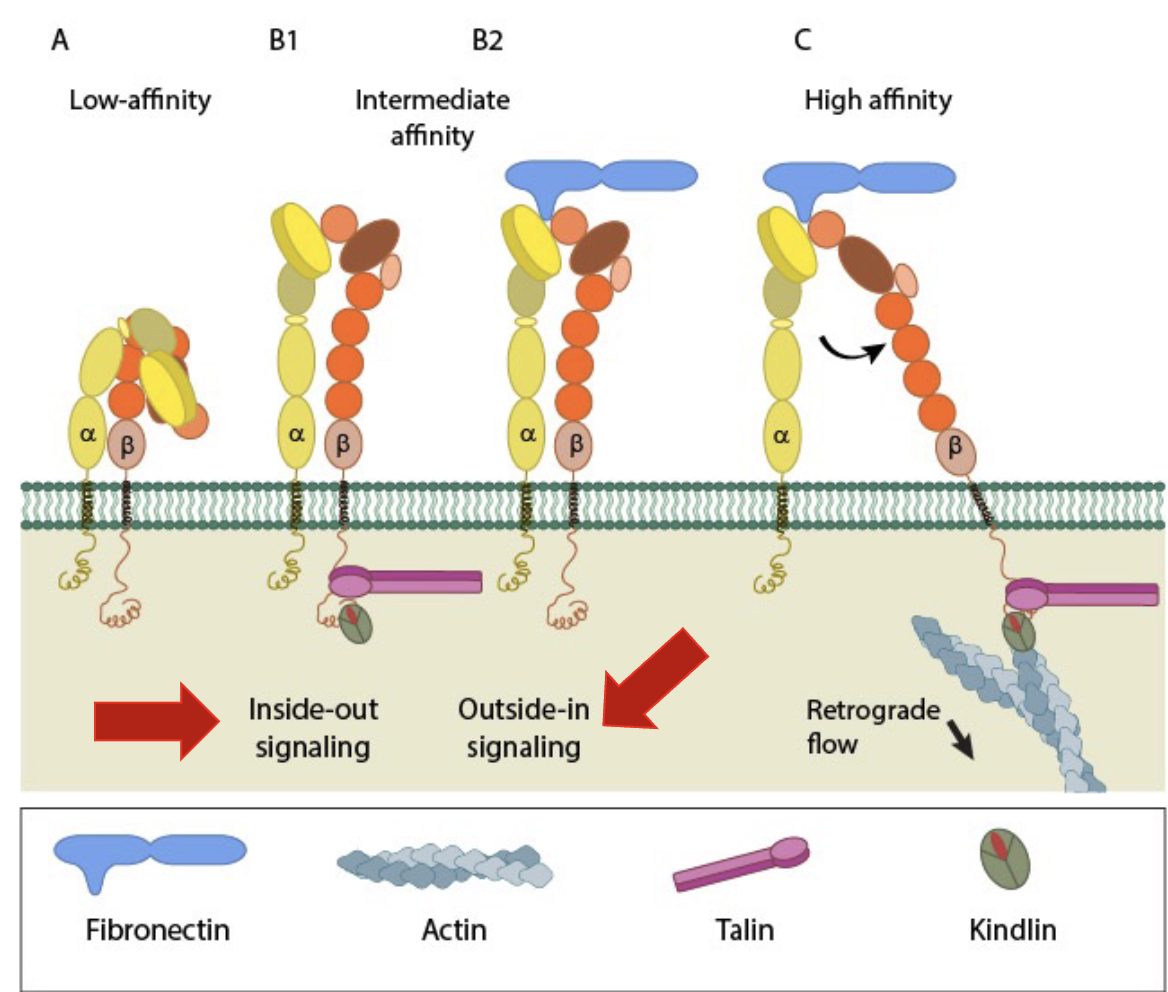
Integrins have 3 conformational states that correspond to binding affinity:
• Bent Closed (low affinity)
• Extended Closed (intermediate affinity)
• Extended Open (high affinity)
What are lectins?
Lectins are carbohydrate-binding proteins that play vital roles in various biological processes, including cell recognition, immune responses, and signaling
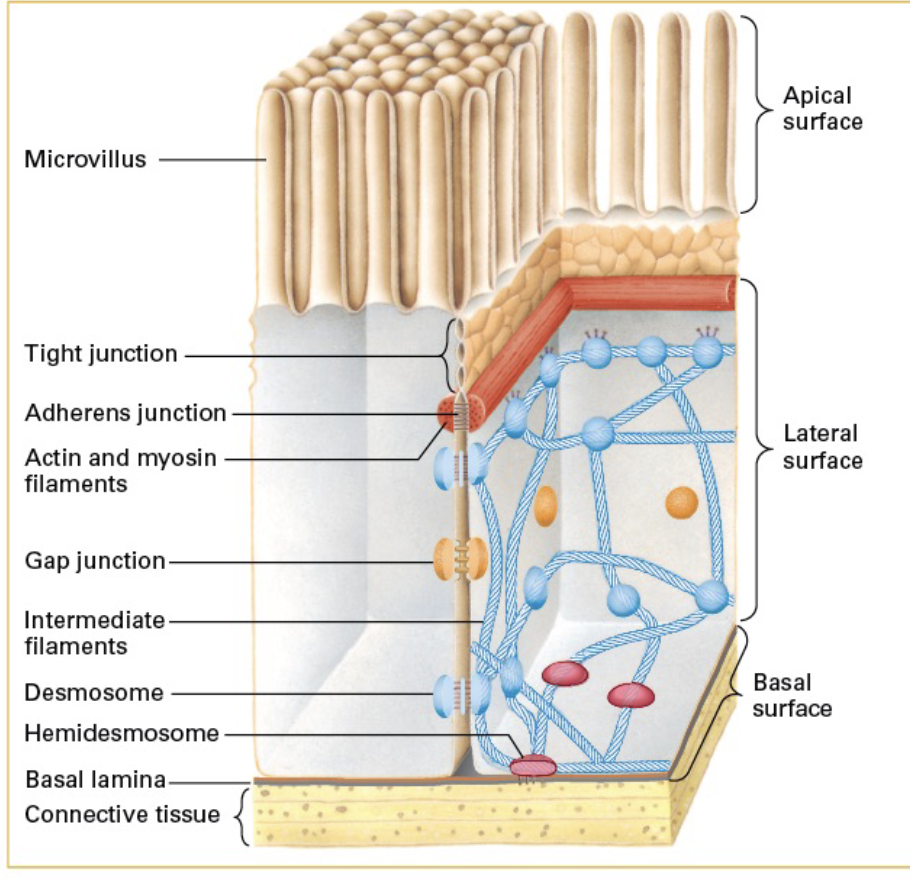
What are cell junctions? List the different kinds.
Cell junctions are structures that connect cells together and help them communicate. TYPES:
(1) Adherens Junctions (2) Desmosomes (3) Hemidesmosomes (4) focal contacts (5) tight junctions (6) gap junctions (7) plasmodesmata
What are adherens junctions?
cell-cell adherence type. Shape, tension,
signaling, force, transmission. Adherens junctions primarily consist of cadherin proteins, which bind to each other on adjacent cells.
*Adherens junctions play a crucial role in connecting adjacent cells, maintaining tissue structure, and enabling coordinated cell movements.
what are desmosomes?
cell-cell adhesion type. Strength,
durability, signaling. Adherence function. Cadherin-dependent. Spot-welding across cells. Connect to keratin filaments (intermediate filaments).
• Blistering diseases are characterized by defects in adhesion:• Cell-Cell and/or • Cell-Substrate
what are hemidesmosomes?
cell-matrix adherence type. Demi/Hemi = half. Adherence to basal lamina. Keratin filament = intermediate filaments. Shape, rigidity,
signaling.
• Spot-welding to the basal lamina (“spot-welding" refers to the strong, localized adhesion between cells that helps maintain tissue integrity under stress).
what are focal contacts? (dont rly need to know)
cell-matrix adherence type. Shape, signaling,
force transmission, cell movement.
What are tight junctions?
cell-cell adhesion type.
Seal off body cavities and restrict diffusion of membrane components. Keep the fluids separated between the epithelial cell layer and the underlying tissues. Form ring structures around the top of the cells. Rings are connected to adjacent cells’ rings.
Many integral membrane proteins are involved in tight junctions;
Tricellulin strengthens tight junctions at the point where three cells’ tight junctions meet.
Junction Adhesion Molecule (JAM) is involved in cell adhesion and the maintenance of tight junctions.
The main proteins involved in tight junctions are occludins and claudins. They help create a seal between cells.
Zona Occludens (ZO) Proteins: ZO-1, ZO-2, and ZO-3 are adapter proteins that connect the transmembrane proteins (like occludins and claudins) to the cytoskeleton inside the cell.
What would happen if tight junctions failed in the intestinal tract?
Tight junctions create a barrier that controls what passes between intestinal cells. If they fail, the permeability of the intestinal epithelium increases, allowing unwanted substances (like toxins, bacteria, and undigested food particles) to pass into the bloodstream.
what are gap junctions?
cell-cell adhesion type. Communication,
small-molecule transport between cells. Plants DONT HAVE gap junctions.
Discovered as cells do not touch 100% laterally. Large complexes found in junctions amidst the gaps
6 protein TM connexon units form hemichannels that connect to adjacent cells. (So, connexons are the building blocks of gap junctions)
~1.2 nm channel for sharing of ions, solutes, and signaling molecules.
*quick localized communication between adjacent cells.
What are tunneling nanotubules?
TNTs facilitate longer-range communication and can transport larger materials between cells that are not in direct contact.
Discovered to appear between adjacent cells
• Short-to-long
• Can transport components such as mitochondria or smaller solutes between them.
TNTs can connect directly to neighboring cells, similar to gap junctions, but their capability to stretch allows them to reach and connect with cells that are further away.
This means TNTs can facilitate intercellular communication beyond the immediate vicinity, enabling a broader range of interactions.
Can transport organelles.
what is the plasmodesmata?
ONLY found in plants. cell-cell adhesion type. Communication,
small-molecule transport between cells.
Cell walls mean almost no CAMs. Plants have plasmodesmata structures that connect adjacent cells.
Plasmodesmata share an ER membrane that lines the structure (the annulus) that is 30-60nm to>1μm in diameter
A narrow tube (desmotubule) is formed with a regulated gap of 2-10nm called the cytoplasmic sleeve.
Allows free transfer of small molecules or regulated transfer of large ones.
Plant Cell Walls INSTEAD OF Intermediate Filaments
• Walls are mostly polysaccharides that form an ECM (~0.2 μm thick)
• Resists turgor pressure (like air filling a balloon)
• Primarily cellulose, hemicellulose and pectin
• Cellulose is synthesized outside the cell membrane, while hemicellulose and pectin are ER-synthesized.
What are elastic fibers?
Found in the ECM in various
tissues. Necessary for tissues with
mechanical strain or deformation (e.g. lungs)
Major component are fibers with an elastin core. Elastin is made of covalently-cross linked tropoelastin monomer aggregates.
Surrounded by microfibrils of collagen which serve as scaffolds.
Tropoelastin is mostly made right before and after birth.
What are Matrix metalloproteinases (MMPs)?
The ECM is dynamic and is often remodeled or degraded. Enzymes are used by cells to remodel or degrade but is mostly done by zinc-dependent matrix metalloproteases (MMPs).
ECM metalloproteases have three major subgroups:
MMPs
23 in humans; remodeling and degradation.
Adisintegrin and metalloproteinases (ADAMs)
Integrin degradation
ADAMs with thrombospondin motifs (ADAMTSs)
Signaling function
What are fibroblasts?
Major secreters of the ECM in us
• Have to be dynamic too
Fibroblasts are crucial in wound healing. They migrate to sites of injury, proliferate, and synthesize ECM comps (collagen,elastin,etc) to help repair and rebuild damaged tissues.
They provide structural integrity to tissues and organs, helping maintain their shape and elasticity.
Cell motility is dynamic (Leukocyte rolling!)
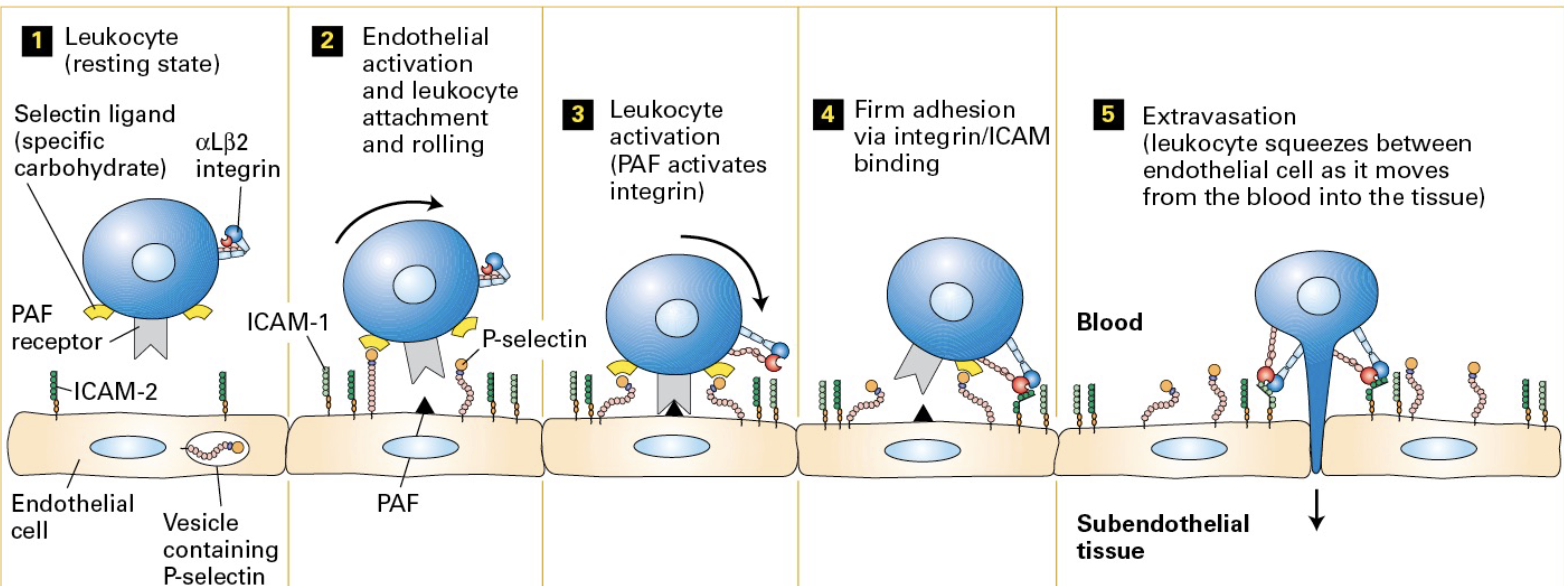
Motility is important for wound healing and immunity.
Inactive immune cells are recruited from the blood to areas of infection and/or inflammation via extravasation.
Inflammatory signals induce endothelial cells to exocytose P-selectin and synthesize PAF so that both appear on the cell surface.
In response to inflammation, signaling molecules (chemokines) are released at the site of injury or infection, activating the endothelial cells.
Activated endothelial cells express selectin proteins on their surface. P-selectin on the endothelial cells binds to carbohydrates on leukocytes weakly causing them to roll along the vessal wall as opposed to adhere firmly.
then PAF receptor binding causes integrin activation on the leukocytes
then the activated integrins binds to ICAM-2 molecules on the endothelial cells. (this binding causes the leukocytes to stop rolling) Leukocytes migrate between endothelial cells out of the bloodstream into the tissue.
What are the three filaments in our cells?
Actin filaments, intermediate filaments, and microtubule filaments.
What are Intermediate Filaments?
One of 3 filaments in our cells
*tensile strength (withstand stress when stretched or pulled without breaking)
Unique properties:
• Great tensile strength - more stable than the other 2
• No intrinsic polarity
• No motor proteins associated
• Not found in all eukaryotic cells
Our genome encodes at least 70 genes for IF
• Grouped into 5 different classes with differing expression.
Dynamic but stable structures.
• Keratin dimer units are acidic/basic pairs - Keratins make hair and nails hard
• Cytokeratins strengthen connection to make a tight skin/dermal layer.
General structure of an intermediate filament;
All have a conserved dimerized 310 aa a-helical rod domain.
10nm thick.
The basic unit is a dimer.
Dimers form anti-parallel tetrads.
Tetrads unite to form protofilaments.
4 protofilaments unite to to form a protofibril.
4 protofibrils unite to form an IF.
Structurally the filaments differ in the non-helical flanks of the conserved domain. Unclear how they form.
Class 1 of Intermediate filaments
made of acidic keratins. found in epithelial cells. used for tissue strength and integrity.
Class two of intermediate filaments
made of basic keratins. found in epithelial cells. used for tissue strength and integrity.
Class three of intermediate filaments
made of Desmin , GFAP, vimentin. Found in (MESODERM cells). Used for sacromere organization and integrity.
Class four of intermediate filaments
made of neurofilaments (NFL, NFM, NFH) found in neurons. used for axon organization.
Class five of intermediate filaments
made from lamins. found in the nucleus. used for nuclear structure and organization.
What are lamins?
Lamins are the meshwork on the inside of our nuclei (nuclear lamina) connecting the membrane and chromatin.
Lamin A provides rigidity. Lamin B associates with receptors and helps attach the nucleus to the cytoskeleton through SUN and KASH domain-containing protein.
What are intermediate-filament associated proteins?
• IFAPs include plakin family members
plakins link IF to the desmosomes and hemidesmosomes
• Other plakins connect IFs to microfilaments and microtubules.
What are actin filaments?
Actin is a major protein component of all cells
It is incorporated into the cells along cell junctions, and throughout the cell’s structures.
Actin, aka the basic unit of thin filaments/microfilaments, is tne of three cytoskeletal filaments . (7-9nm)
10% of total protein in a muscle cell. 1-5% of total protein in a non-muscle cell
Filaments can form and be undone constantly
Filaments can also be stabilized for long periods of time.
Humans have 6 actin genes
Actin filament functions
Actin is employed in cells in various structures
It is involved in various functions from structure, to movement, to “eating” and cell reproduction
3 general categories based on charge.
alpha-actin – in muscle fibers
beta-actin – e.g., in the leading edge
gamma-actin – e.g., in stress fibers
Under the right conditions, actin can polymerize by itself
In cells, many proteins are associated with actin and perform various functions such as modifying actin by nucleating, stabilizing, severing, capping, etc.
List the types of actin-associated proteins.
Profilin, Cofilin, Thymosin-beta4, CapZ, Tropomodulin, Gelsolin, Formins (Arp2/3) , (Fimbrin, alpha-actinin, filamin, spectrin), ankyrin+dystrophin, myosins, nebulin, tropomyosin.
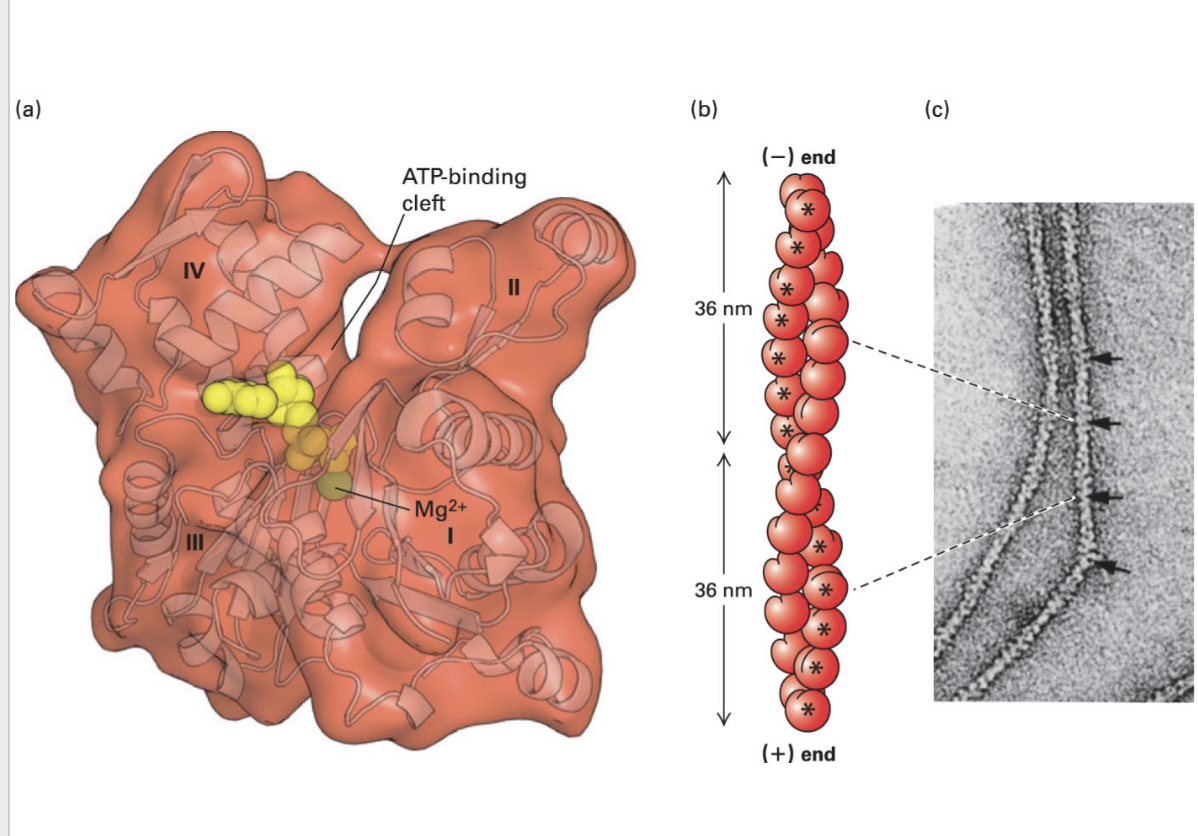
Actin Monomers Structure
Actin exists as globular monomers (G-actin) in cells
[Binds ATP and Mg2+ in a cleft. Is an ATPase]
Polymerized actin forms filamentous actin (F-actin) [2 helical structures wrapped around one another]
F-actin form 36 nm repeat structure
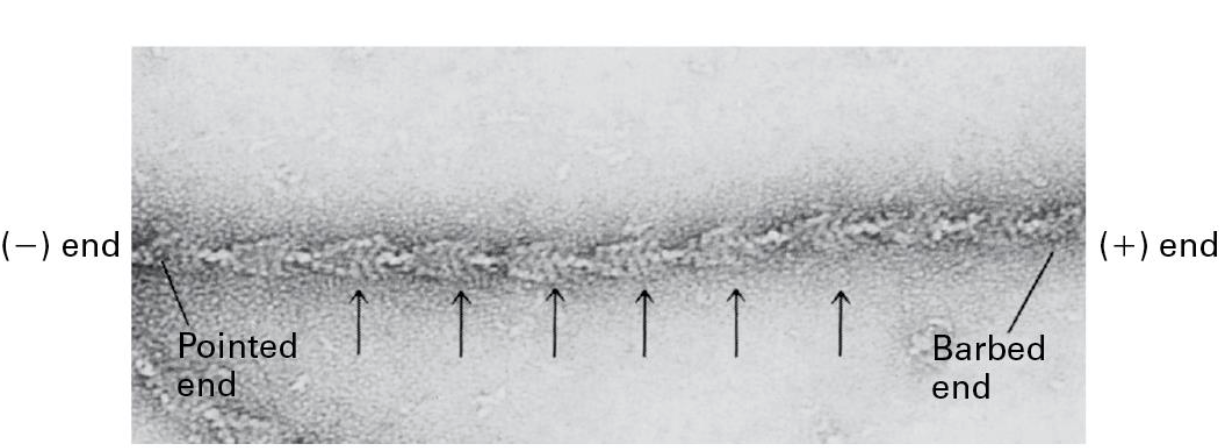
Actin filaments are polarized!
Myosin S1 region can coat F-actin
• Actin-binding region of Myosin
• Forms arrow-like structures
• (-) Pointed end
• (+) Barbed end
(-) end has the cleft exposed to the solution
(+) end has the cleft contacting other subunits

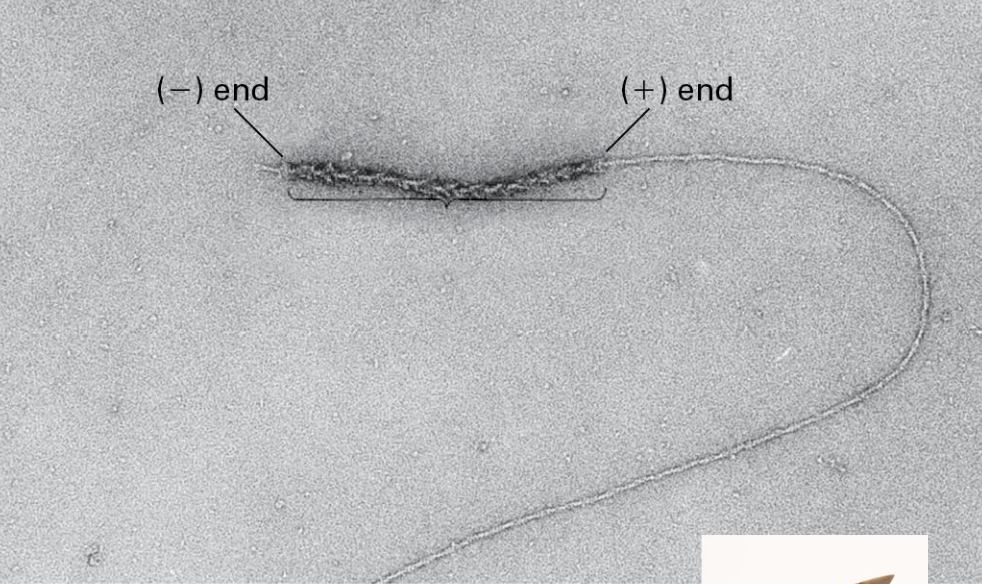
Actin Filament Dynamics are Lopsided BC?
Actin monomers are added more readily to the (+) end than the (-) end of a filament
To form filaments G-actin-ATP units are added to both ends. (+) end addition is almost 10x greater than the (-) end.
THIS IS BC:
Actin filaments require a critical concentration (Cc)
of free G-Actin-ATP molecules and [Cc] required
in the (+) end is much lower than it is for -.
What is treadmilling?
It is seen at steady state the (-) end loses monomers while new ones are added to the (+) end
How does actin form filaments, what are the three steps?
(1) nucleation (2) elongation (3) steady state;
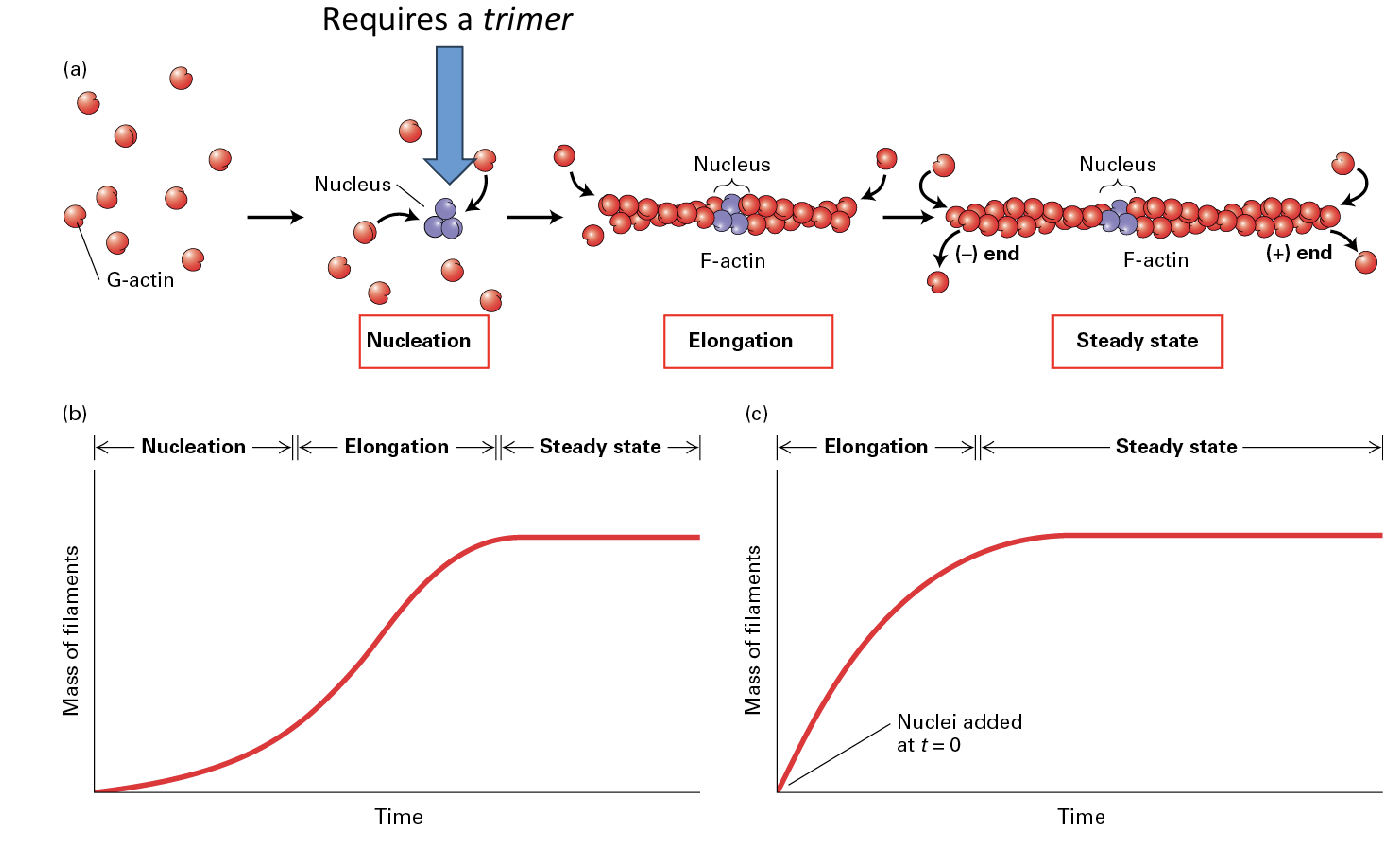
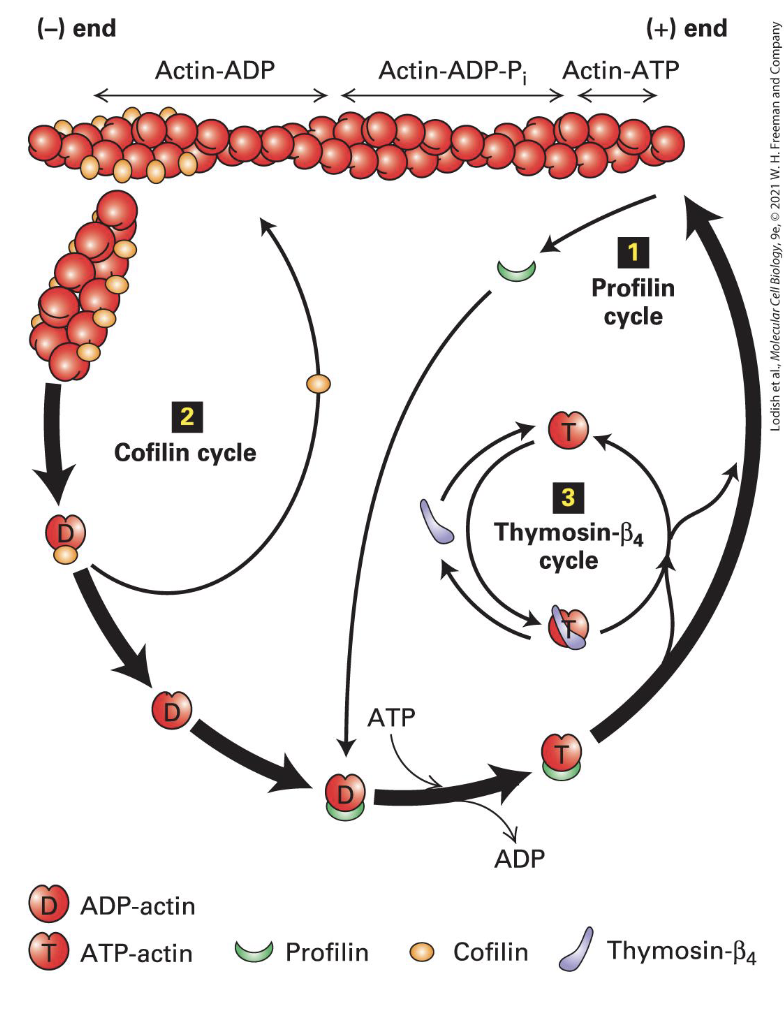
Recylcing Actin
Profilin binds G-actin lost from filaments and opens the cleft enhancing loss of ADP
Profilin-ATP-actin complex binds the (+) end
Cofilin binds 2 ADP-actin towards the (-) end, causes a twist and a break
Generates more (-) ends for disassembly
Thysomin-beta4 binds free ATP-actin (G-actin) to keep it from forming F-actin
Forms dynamic equilibrium with the free subunits.
Actin exists in two forms:
G-actin (globular actin): The monomeric, free form of actin.
F-actin (filamentous actin): The polymerized form that makes up the structural filaments.
Actin filaments can rapidly grow (polymerize) and shrink (depolymerize) in response to cellular signals and needs.
Proteins like profilin promote the addition of G-actin to the growing ends of filaments, while cofilin facilitates disassembly by binding to ADP-actin at the (-) ends.
When actin filaments are no longer needed or damaged, they disassemble into G-actin. This G-actin can then be recycled back into the actin pool, where it can be reassembled into new filaments as needed.
Thymosin-β4 binds to free G actin, preventing it from assembling unnecessarily.
Formin and Arp2/3 complex, help nucleate new filament formation when required.
Recycling allows cells to quickly respond to changes in their environment.
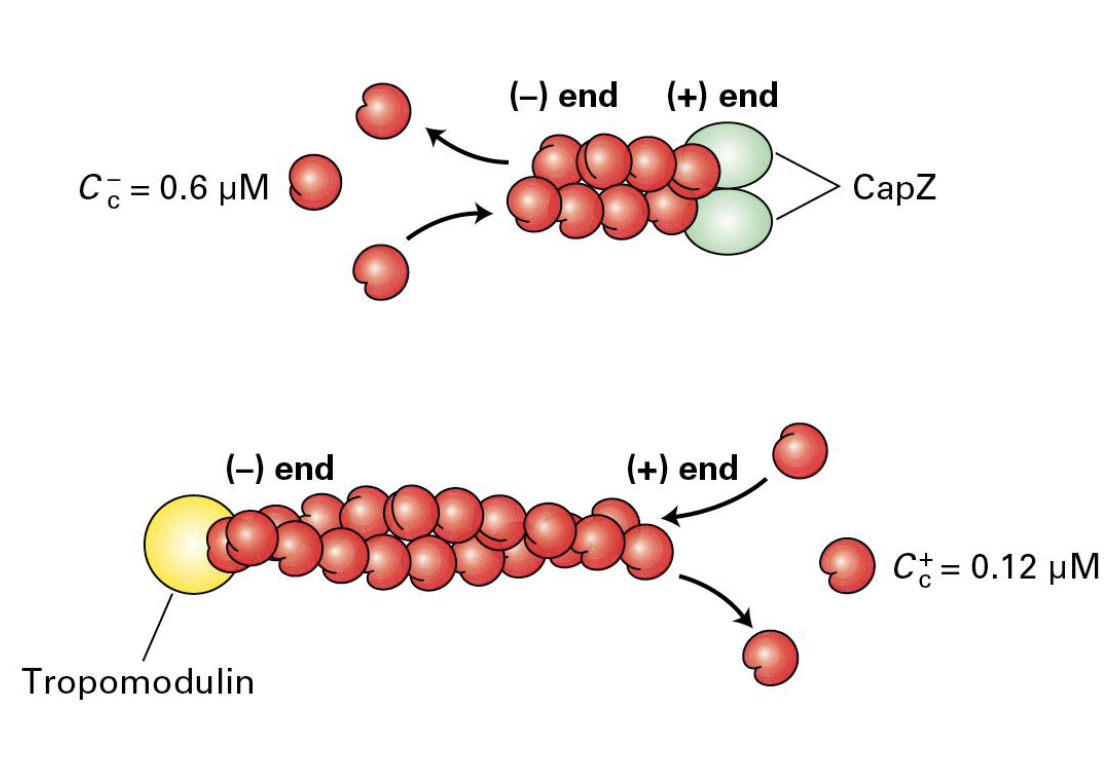
Capping Proteins (CapZ and Tropomodulin)
These are used in order to regulate the formation and length of filaments/dynamics.
CapZ binds to the (+) end and cells regulate CapZ ability in order to regulate CapZ ability to stop growth. (CapZ is formed from two related proteins).
Tropomodulin binds the (-) end and can module filament assembly and disassembly (mostly found where actin needs to be stabilized).
Form Homology Domains (FH1 and FH2) in Formin
FH1 domains bind profilin to increase ATP-actin. this in turn enhances the assembly and stability of actin filaments.
FH2 domain forms a ring that rocks back and forth which promotes addition of ATP-actin to the positive end
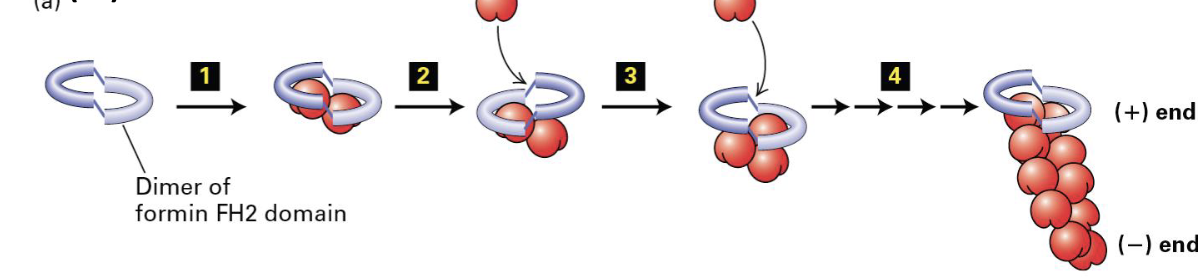
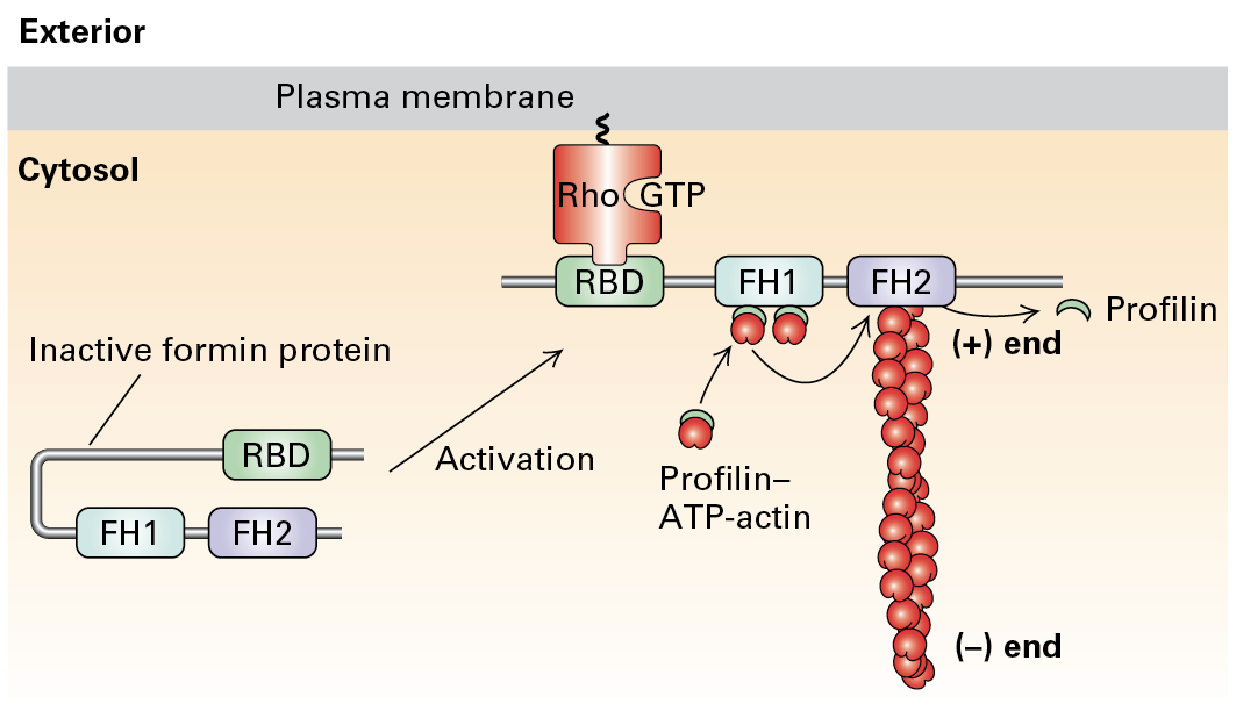
Formin Protein
Actin nucleation and filament formation can be under the control of signal pathways.
Profilin accelerates nucleation and creation of long F-actin upon receptor-mediated signaling activation which activates the protein promoting F-actin.
F-Actin assembly
Two major classes of actin-nucleating proteins help in the assembly of F-actin in cells
• Formin protein family members:Long Filaments
• Arp2/3 complex: Branched filaments
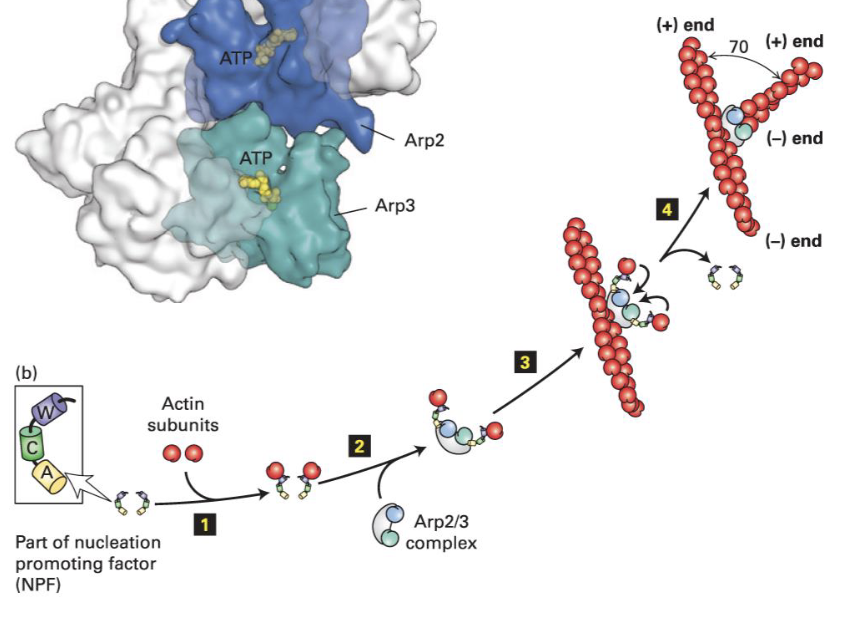
What is the (actin-related protein) ARP2/3 complex?
ARP2/3 is a protein complex. It causes nucleation and creation of branched F-actin after activation by a nucleation-promoting factor (NPF).
The complex binds to existing F-actin and promotes branch formation.
WASp (Wiskott-Aldrich syndrome) Protein
It normally is inactive. Activation of signaling pathways turned on by receptors causes its activation.
Activation in turn recruits Arp2/3 near the membrane.
Listeria Monocytogenes
Intracellular parasite causing food-borne illness.
Comet tails; ActA protein (listeria) mimics an NPF and activates and recruits Arp2/3
Actin polymerization propels the bacteria through the cell.
Actin Filaments and Force
Endocytosis brings in materials from outside the cell
Bursts of Arp2/3 activation recruits other factors (e.g., Myosin VI) that can help power this energetically unfavorable membrane dynamic.
Phagocytosis w/Actin as an Immune Response
Actin is involved in phagocytosis (”cell eating”).
Several immune cells function by “eating” disease-causing agents.
Opsonized pathogens are immobilized by the
cell and receptor-mediate signaling activates
local Arp2/3 activation and network formation.
Creates a contractile network that leads to the
isolation of the pathogen and subsequent destruction.
Actin-cross linking
acting-cross linking proteins mold actin filaments into diverse structures , they are organized and cross-linked by fimbrin proteins.
Erythrocytes + actin!
Erythrocytes need an actin network to withstand stress and maintain their shape. Proteins like alpha/beta spectrin anchor this network to the cell membrane, providing the necessary support to prevent the cells from breaking apart and ensuring their proper function.
Actin Filaments and Muscle (w/myosin)
All family members (of myosin) are motor proteins that can bind to Actin.
Convert chemical-energy to mechanical work.
There are 40 myosin genes in the human genome.
In laboratory settings, researchers often use immobilized myosin S1 to study the mechanics of muscle contraction and actin dynamics. By applying ATP, they can observe how actin filaments move relative to the fixed myosin.
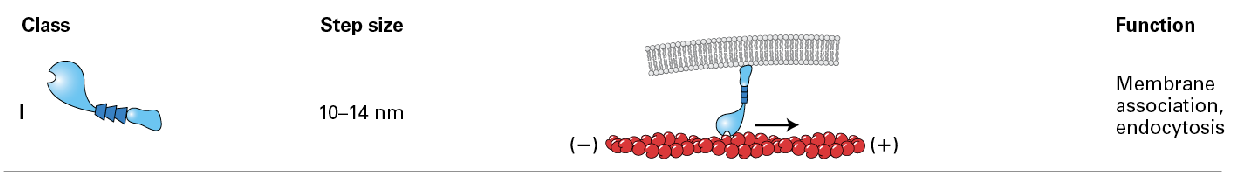
Myosin 1
only one with a single head domain and it is associated with the membrane.
Myosin 2
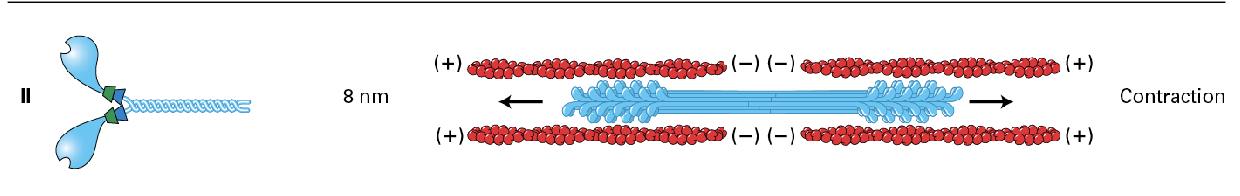
mostly in muscle (thick fibers) but also non-muscle in less organized fashion, used for
contraction
(moves about 8nm)
Myosin 5 (V)
motor protein to hall things towards the membrane
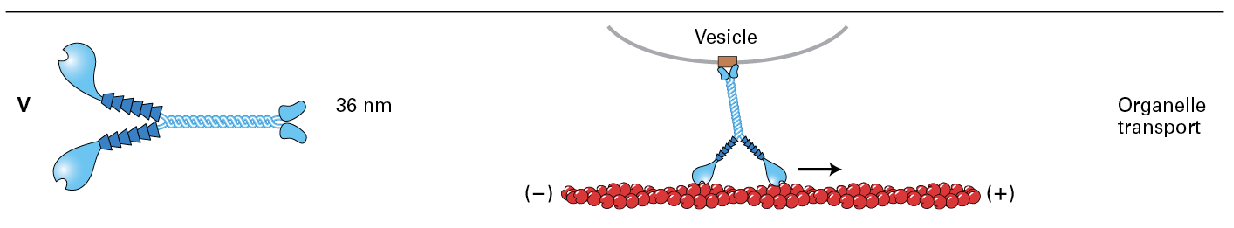
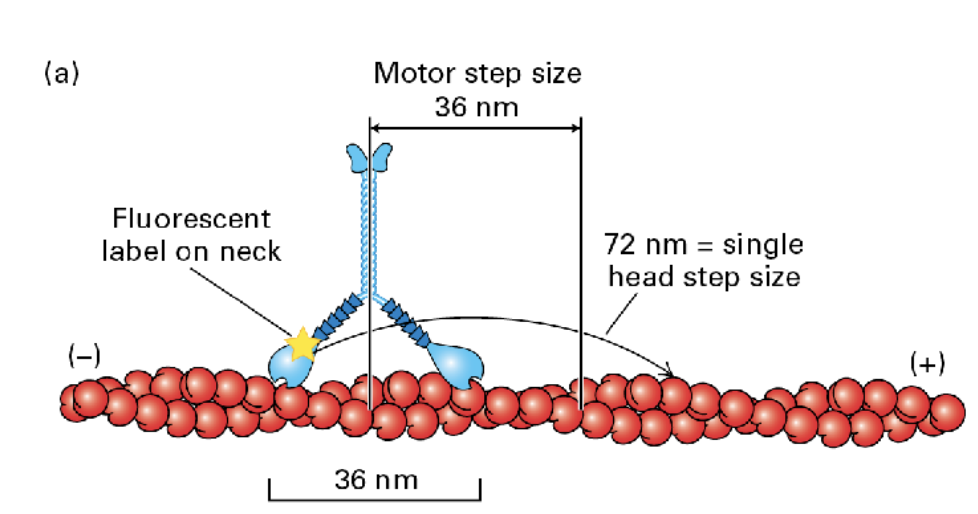
Myosin 5 moves take longer 72nm steps hauling cargo 36 nm at a step. (motor step size = 36 nm whereas single head step size is 72nm)
Myosin 6 (VI)
only member that moves towards the negative end, moving things away from the membrane
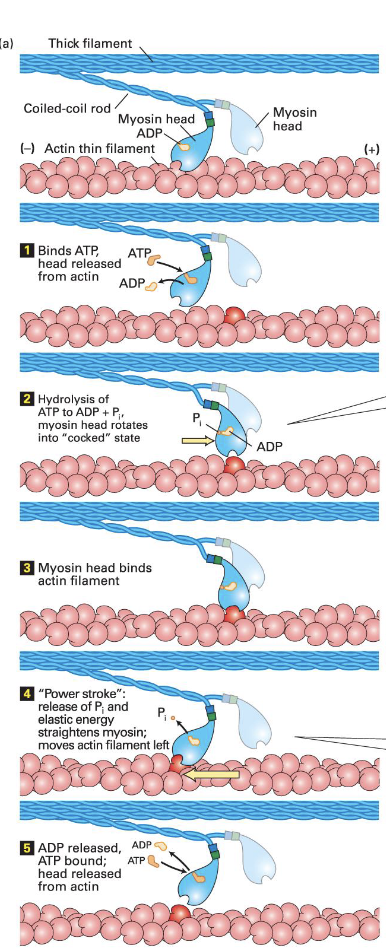
Power stroke; Myosin movement (steps)
Myosin-ADP binds very well to f-actin
ATP binds releasing myosin from F-actin
Hydrolysis of ATP cocks the head domain
Head domain binds actin
Release of Pi moves it back into its original position
In muscle 10% of the myosin is contacting f-actin when activated.
Myosin steps
The step size of the myosin head is relative to the length of the neck domain
• E.g., longer legs = bigger step
Myosin II moves about 8 nm
Myosin V takes longer 72 nm steps, hauling cargo 36 nm at a step
Sacromere Units/Muscles Composition;
Myosin II thick filaments alternating with F-actin thin filaments attached to the Z disk → Z disk → sarcomere → myofibril → multi-nucleated muscle cells → muscle fibers → muscle
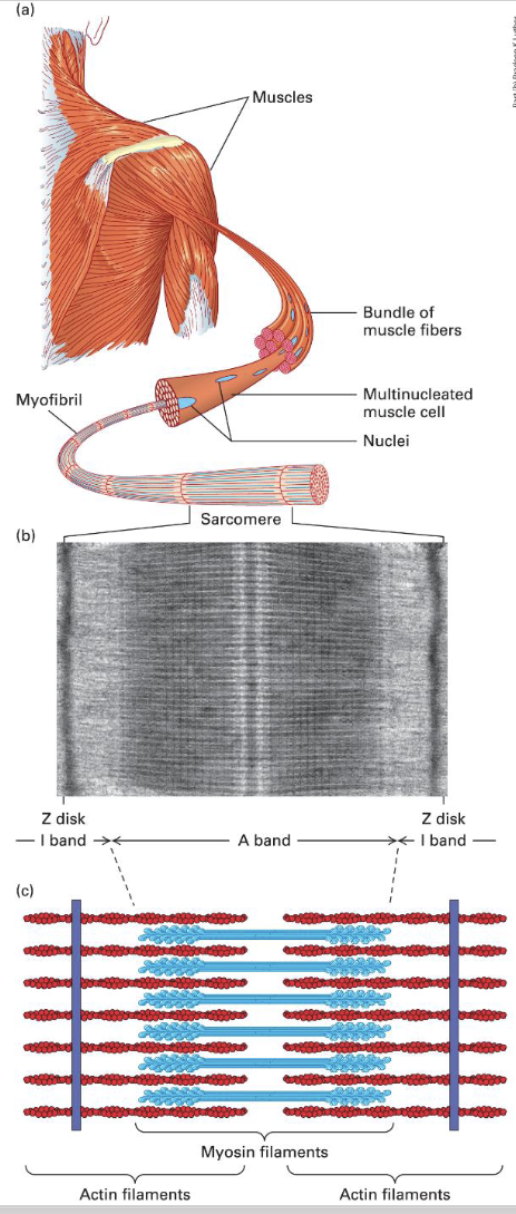
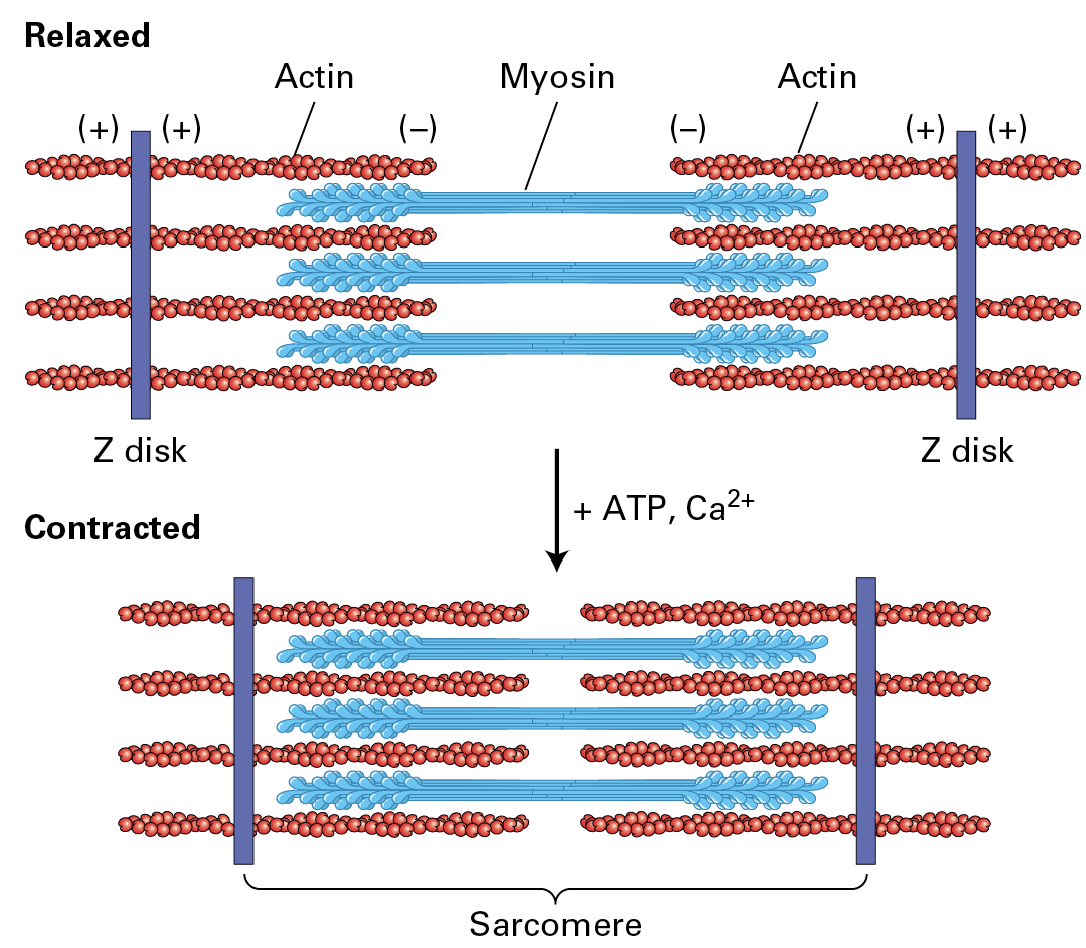
Relax / Contract of Muscle Cells (sarcomere shortening):
Thick filaments are bipolar with heads out towards the Z disks (the blue heads) F-actin is attached to the Z-disk.
During contraction, the myosin II heads grab onto the actin and pull them in so the distance between the (-) gets shorter and (+) comes in more.
Controlled by ATP availability and Ca2+ influx.
How is Ca2+ released for skeletal muscle contraction?
The sarcoplasmic reticulum (SR) holds a high store of Ca2+ for skeletal muscle regulation. Neural impulse travels and pens voltage-gated Ca2+ channels in the SR. The release of Ca2+ allows muscle contraction by binding Troponin. Troponin shifts Tropomyosin.
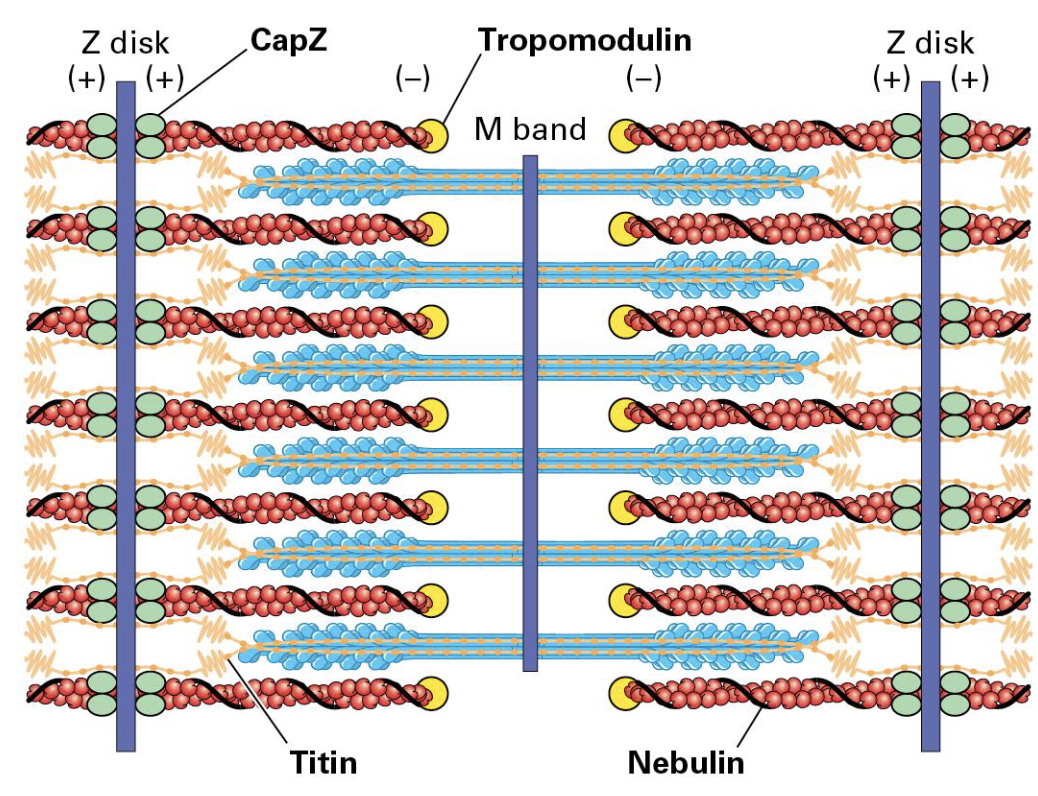
Accessory Proteins
Keeping muscle intact is work
CapZ helps bind F-actin (+) and Tropomodulin (-)
Giant elastic Titin protein binds Z disk and extends toward the M band on either side. (Holds the thick filaments in place.)
Giant nebulin protein wraps around the F-actin.
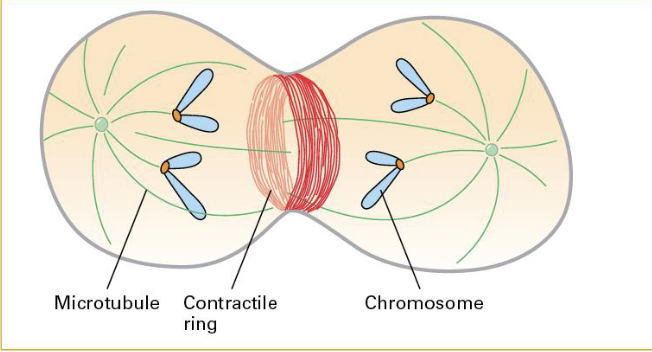
Actin/Myosins used for the Contractile Ring (cytokinesis).
A contractile ring forms after chromosomal partition. Eventually pinches off (this is via help of actin/myosin 2)
Contraction in Smooth Muscle
Smooth Muscle Contraction is controlled by signaling rather than by Ca2+ availability.
Myosin is inactive during relaxation and phosphorylation activates it forming filaments that can induce contraction in smooth muscle.
Dephosphorylation relaxes it again.
Actin Filaments in Movement;
Actin is utilized in cell motility.
Four major steps characterize it; (1) extension (2) adhesion (3) translocation (4) de-adhesion and endocytic recycling.
• Some do this slowly (e.g., fibroblasts) Others do this quickly (e.g., leukocytes)
• Dynamic adhesion to the ECM is required.
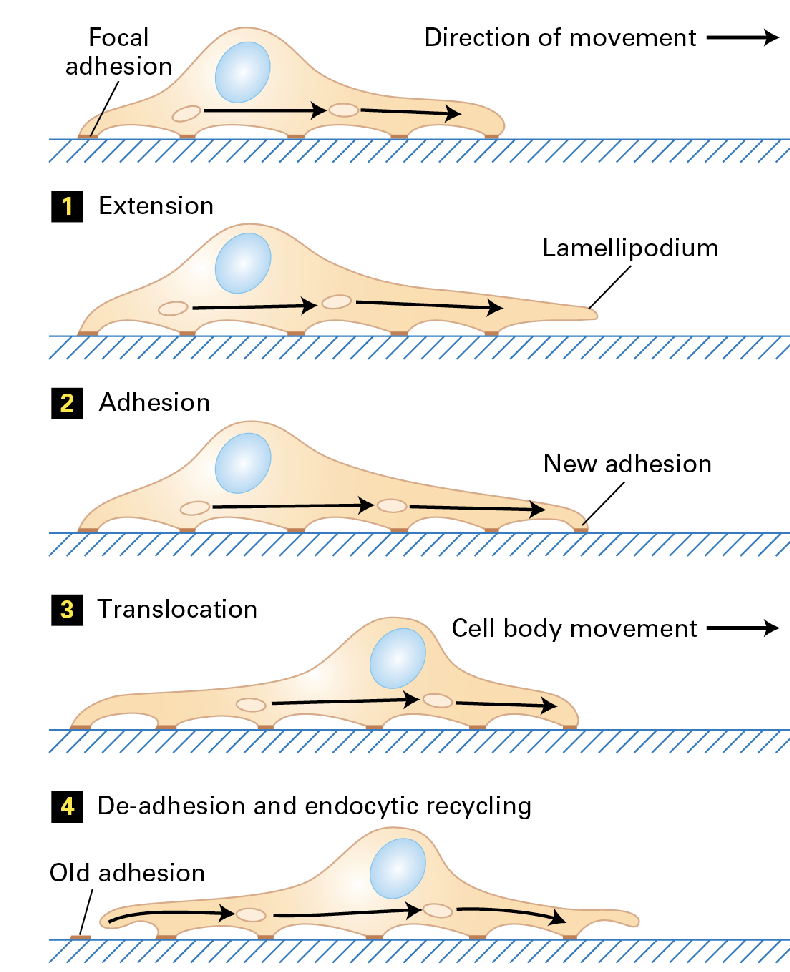
The Leading Edge
The leading edge is the force that pushes against the membrane extending it forward. Arp2/3 gives cells that force at the right place.
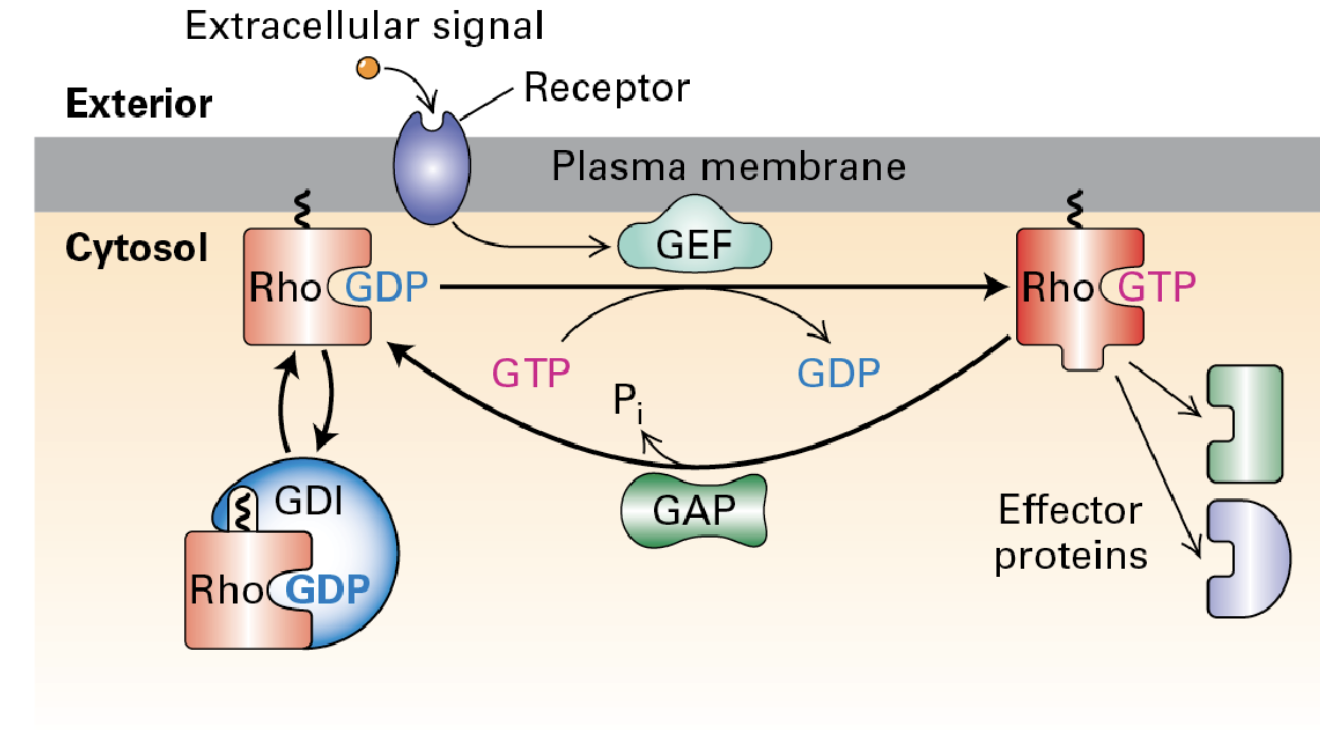
Actin Signaling Pathway
The actin cytoskeleton can be regulated by signaling pathways involving Rho family G-proteins. Guanine nucleotide dissociation inhibitors (GDI) keep these proteins inactive until they are recruited and activated by GEFs through receptor signaling. Once activated, GTPase-activating proteins (GAPs) help deactivate them.
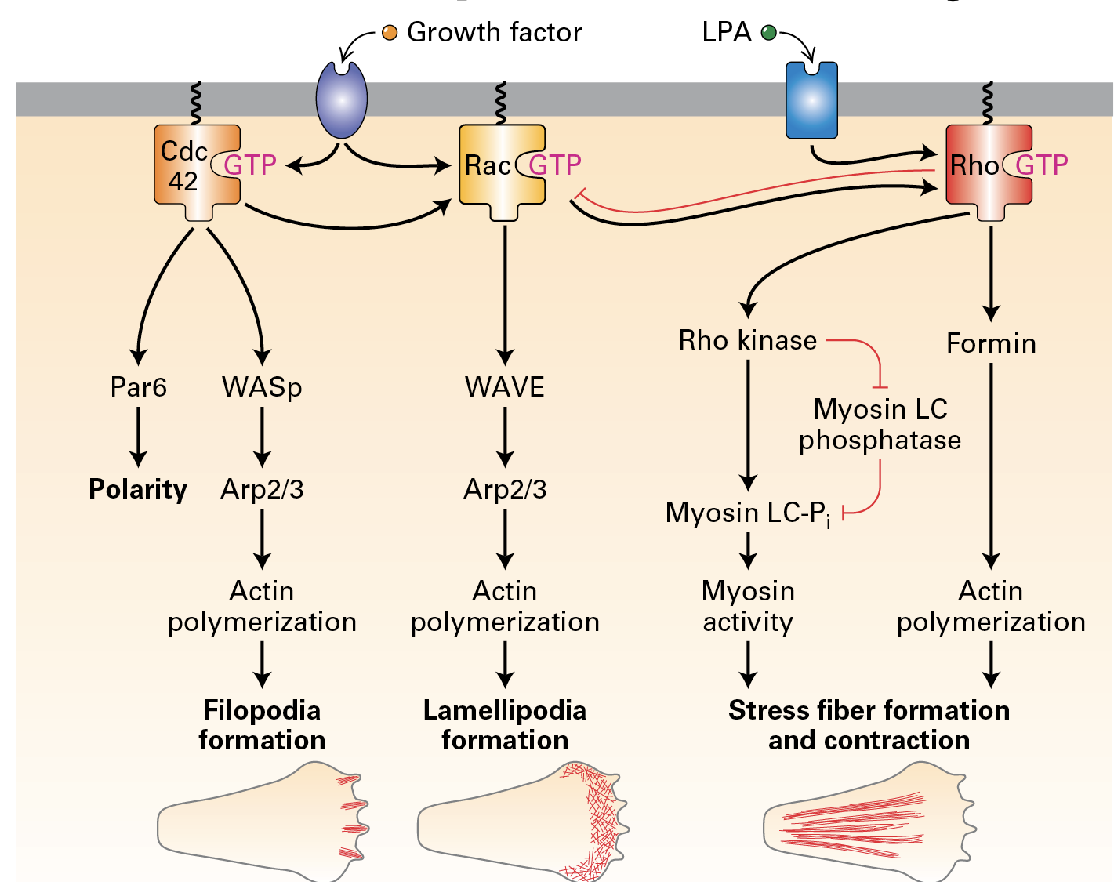
Dominant-active (GTP-bound) mutants
cause several different phenotypes in cells
• Membrane ruffles
• Stress fiber formation
• Filopodia creation
THREE IMPORTANT PATHS! (Filopedia formation, lamellipodia formation, stress-fiber formation + contraction).
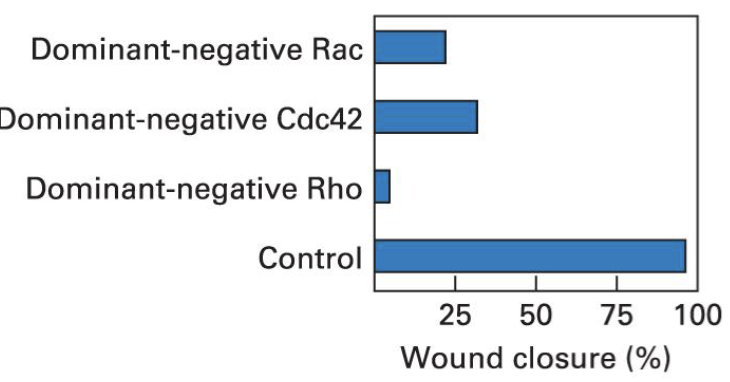
The Scratch Assay
Dominant negative mutants are modified proteins that interfere with the function of the normal protein.
A scratch assay is used to study cell migration. In this assay, a "scratch" or gap is created in a layer of cells, and the migration of cells into this gap is observed over time.
Cell motility is vital in processes like wound healing, immune responses, and tissue development.
If introducing a dominant negative form of a protein significantly impairs the ability of cells to migrate into the scratch area, it demonstrates that the normal function of that protein is crucial for cell movement.
Lamellapodia
considered the leading edge, they are crucial for cell movement. They extend the cell forward and help it detach from surfaces behind it, enabling efficient migration.
a dynamic coordination of the three signaling paths.
Actin filament assembly and treadmilling in the leading edge.
CdC42 activation at the front. → Front: RAC activation leading to Arp2/3 activation. → Back: Rho Activation leading to myosin 2 activation.
Migration (Chemotaxis);
It’s the directed movement of cells toward the source of a concentration gradient. This is important in wound healing.
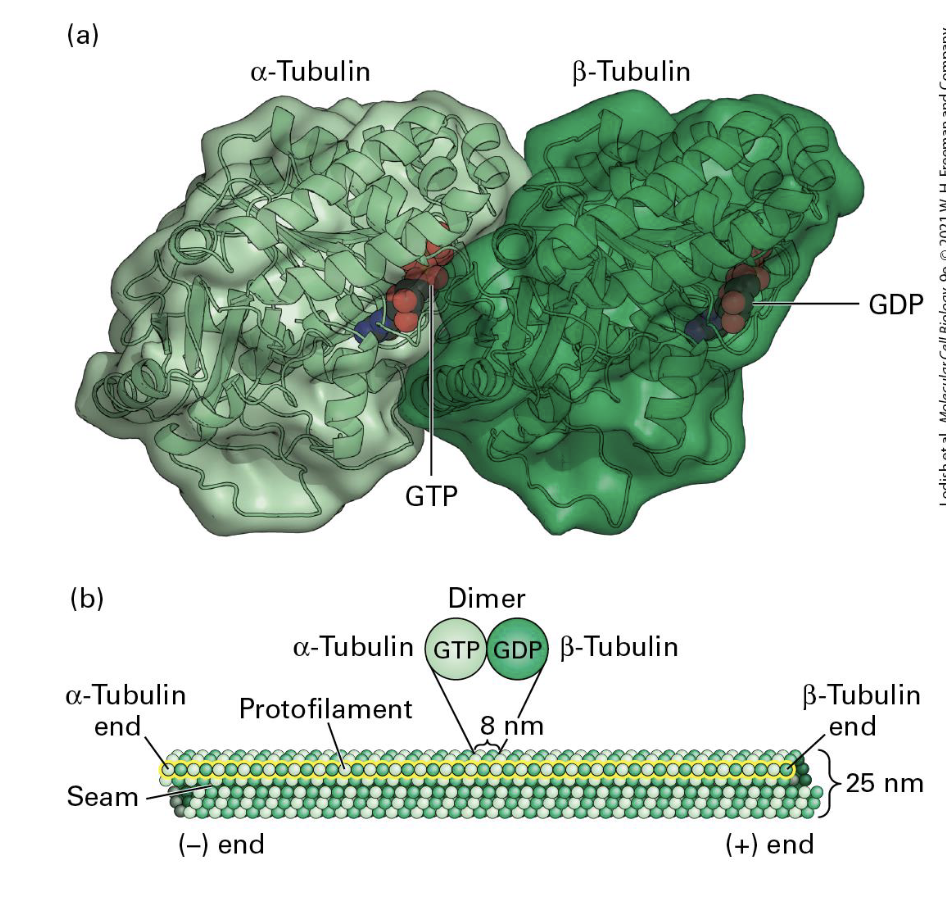
Microtubules
alpha-beta tubulin binds GTP , rigid and not easily bent. polarized → tracks for kinesins and dyneins → organization and long-rage transport for organelles.
Microfilaments (actin)
actin binds ATP. Form rigid gels, networks, and linear bundles. → polarized → tracks for myosin → contractile machinery and network at the cell cortex.
Intermediate Filaments
IF subunits DONT bind a nucleotide. great tensile strength. Assembled onto pre-existing filaments → less dynamic → unpolarized → no motors → cell and tissue integrity.
Microtubules function:
They form highways for the organelles and transport of substances in our cells.
They form important parts of structures such as cilia and axons.
Microtubules Structure:
alpha and beta tubulin form dimers
Dimer = basic unit of microtubules
Similar yet distinct structures, binding GTP
One polarized filament = protofilament
13 protofilaments = microtubule
Filaments come in;
Singlet (13 units - found in cyotplasm)
Doublet (13 + 10 units - found in cilia + flagella)
Triplet (13+10+10 units -found in primary cilia (basal body) + centrioles).
Microtubules are long and extended but can form branch structures.