The Structure and Function of Macromolecules
Macromolecules
- Are large molecules composed of smaller molecules
- Are complex in their structure
- Most macromolecules are polymers, built from monomers
- Four classes of life’s organic molecules are polymers
- Carbohydrates
- Proteins
- Nucleic acids
- Lipids
- A polymer
- A long molecule consisting of many similar building blocks called monomers
- Specific monomers make up each macromolecule
- E.g. amino acids are the monomers for proteins
The Synthesis and Breakdown of Polymers
- Monomers form larger molecules by condensation reaction called dehydration synthesis
- Polymers and disassemble by hydrolysis
- Addition of water molecules
- Although organisms share the same limited number of monomer types, each organism is unique based on the arrangement of monomers into polymers
- An immense variety of polymers can be built from a small set of monomers
Carbohydrates
- Serve as fuel and building material
- Include both sugars and polymers
- Starch, cellulose, etc.
Sugars
- Monosaccharides
- Are the simplest sugars
- Can be used for fuel
- Can be converted into other organic molecules
- Can be combined into polymers
- Can be linear
- Can form rings
- Disaccharides
- Consist of two monosaccharides
- Are joined by a glycosidic linkage
- Polysaccharides
- Are polymers of sugars
- Serve many roles in organism
- Storage Polysaccharides
- Starch
- Is a polymer consisting entirely of glucose monomers
- Is the major storage form of glucose in plants
- Glycogen
- Consists of glucose monomers
- Is the major storage form of glucose in animals
- Cellulose
- Is a polymer of glucose
- Has different glycosidic linkages than starch
- Difficult to digest
- Cows have microbes in their stomach to facilitate this process
- Chitin
- Is found in the exoskeleton of arthropods
- Can be used as surgical thread
Lipids
- Are the one class of large biological monomers that do not consist of polymers
- Share the common trait of being hydrophobic
Fats
Constructed from two types of smaller molecules
A single glycerol and usually three fatty acids
Vary in length and number and location of double bonds they contain
Saturated fatty acids
Have the maximum number of hydrogen atoms possible
Have no double bonds
Unsaturated fatty acids
Have one or more double bonds
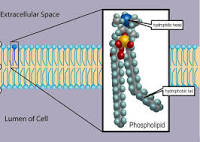
Phospholipids
Have only two fatty acids
Have a phosphate group instead of third fatty acid
Structure
Consists of a hydrophobic “head” and hydrophobic “tails”
Results in a bilayer arrangement founds in cell membranes
Steroids
- Lipids characterized by a carbon skeleton consisting of four fused rings
- Cholesterol
- Found in cell membranes
- Is a precursor for some hormones
Proteins
- Proteins have many structures, resulting in a wide range of functions
- Proteins do most of the work in cells and acts as enzymes
- Proteins are made of monomers called amino acids
- Enzyme
- Type of protein that acts as a catalyst, speeding up chemical reactions
- Polypeptides
- Polymers of amino acids
- A protein consists of one or more polypeptides
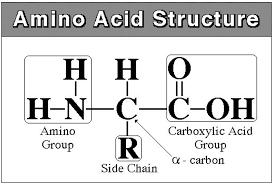
- Amino acids
- Are organic molecules possessing both carboxyl and amino groups
- Differ in their properties due to differing side chains, called R groups
- Linked by peptide bonds
Protein Conformation and Function
- A protein’s specific conformation (shape) determines how it functions
Four Levels of Protein Structure
- Primary structure
- Unique sequence of amino acids in a polypeptide
- Secondary structure
- Folding or coiling of the polypeptide into a repeating configuration
- Includes the a helix and β pleated sheet
- Tertiary Structure
- Overall three-dimensional shape of a polypeptide
- Results from interactions between amino acids and R groups
- Quaternary structure
- The overall protein structure that results from the aggregation of two or more polypeptide subunits
Sickle Cell Disease
- Results from a single amino acid substitution in the protein hemoglobin
What determines Protein Conformation
- Protein conformation depends on the physical and chemical conditions of the protein’s environment
- Temperature, pH, etc.
- Denaturation is when a protein unravels and loses its native conformation
The Protein Folding Problem
- Most proteins
- Probably go through several intermediate states on their way to a stable conformation
- Denaturated proteins no longer work in their unfolded conditions
- Proteins may be denaturated by extreme changes in pH or temperature
- Chaperonins
- Protein molecules that assist in the proper folding of other proteins
- X-ray crystallography
- Used to determine a protein’s three-dimensional structure
Nucleic Acids
- Store and transmit hereditary information
- Genes
- Are the units of inheritance
- Program the amino acid sequence of polypeptides
- Are made of nucleotide sequences of DNA
- DNA
- Deoxyribonucleic acid
- Stores information for the synthesis of specific proteins
- Found in the nucleus of the cell
- Functions
- Directs RNA synthesis
- Transcription
- Directs protein synthesis through RNA
- Translation
- Structure
- Nucleic acids exist as polymers called polynucleotides
- Each polynucleotide
- Consists of monomers called nucleotides
- Sugar + phosphate + nitrogen base
- Nucleotide monomers
- Made up of nucleosides (sugar + base) and a phosphate group
- Nucleotide polymers
- Are made up of nucleotides linked by the -OH on the 3’ carbon of one nucleotide and the phosphate on the 5’ carbon of the next
- Gene
- The sequence of bases along a nucleotide polymer
- DNA double helix
- Have two polynucleotides that spiral around an imaginary axis
- Form a double helix
- Consists of two antiparallel nucleotide strands
- A, T, C, G
- The nitrogenous bases in DNA
- Form hydrogen bonds in a complementary fashion
- A with T only
- C with G only
DNA and Proteins as Tape Measures of Evolution
- Molecular comparisons
- Help biologists sort out the evolutionary connections among species