Foundations (VMED 5275): Cell Degeneration, Necrosis, Injury, and Death
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
the cell functions due to
genetic programs of metabolism
differentiation
specialization
constraints of neighboring cells
availability of metabolic substrates
cellular adaptations
severe physiologic stresses and pathologic stimuli, causing physiologic and morphologic changes
cellular adaptation will cause
new but altered steady states
preserve viability of the cell
modulate fxn.responding to stimuli
cellular injury results when
the limits of adaptive response to a stimulus are exceeded or if a cell is exposed to an injurious agent or stress
cell injury is reversible to a certain point
true
if stimulus persists or is severe enough
cell reaches point of no return, irreversible cell injury and cell death
standard cells organelles
synthesis of lipids, proteins, CHOs
energy production
transport of ions and other molecules
cells respond to homeostatic changes
adaptation
examples of cellular adaptations
increasing muscle mass
increasing p450 fxn. in oxidation expression in hepatocytes
cells respond by increasing/decreasing content of organelles
atrophy
reduction in mass of tissue or organ
hypertrophy
increase in the size of cells, resulting in enlargement organs
hyperplasia
increased number of cells in an organ or tissue
metaplasia
transformation or replacement of one adult cells type with another
cell swelling
acute change in reversible cell injury
cell enlargement
seen in chronic sublethal injury
signal of hypertrophy
increased proteins content of cells
increased organelle number
myofibrils
MITOCHONDRIA
endoplasmic reticulum
hypertrophy may not be always advantageous to the animal
true
list 4 changes as cellular adaptations
atrophy
hypertrophy
metaplasia
hyperplasia
increase in size of cells
hypertrophy
cell injury
loss of the ability to maintain the normal or adapted homeostatic state
unable to balance/regulate internal environment
extent of injury varies
severity of stimulus
type of cell involved
matabolic state a time of injury
cell degeneration
reversible cell injury\ can be unclear to differentiate between adaptation
histologic slides may not be able to
determine if a cell is getting worse or better
may not be able to determine if the injury would have been reversible or not
morphologic chnages lag behind functional changes
true
hallmarks of cells degeneration (cellular accumulations)
cell swelling
fatty change
glycogen accumulation
lipofuscin and ceroid = oxidized product form membrane to lipids=yellow to brown
hyaline changes = dense, homogenous, glossy, translucent many causes protein leakage
amyloid
mucinous changes = gelatinous, semisolid, slimy, clear, stringy
calcification
gout
cholesterol crystals
inclusions
reduction in the mass of a tissue or organ, loss of cells, reduction in size of cells
atrophy
disuse atrophy
decrease workload
dystrophic atrophy
decreased nutrition
endocrine/hormonal atrophy
loss of hormonal stimulation
anemic atrophy
decreased blood
neural atrophy
loss of innervation
pressure atrophy
tissue compression
physiological atrophy
senile atrophy
cellular atrophy occurs when
reversible damage occurs
reduced functional capacity
continue to control internal environment and produce sufficient energy for metabolic stsate
adrenal glands - addisonian crisis can occur when
sudden withdrawal of corticosteroids
prolonged cellular atrophy may lead to
death of some cells
loss of muscle cells
hypertrophy
increase in organ cell size
increases organ size without cellular proliferation
hyperplasia
increase in number of cells in an organ
in the heart, hypertrophy does not change underlying problem such as valvular stenosis
true
heart hypertrophy can cause
decreased ejection volume and may eventually end up in organ failure
cellular hyperplasia
increase in number of the principal cells of a tissue or organs
cellular hyperplasia can only occur in a cell population that is capable of mitosis
true
epithelial cells, hepatocytes is an example of cells where hyperplasia can occur
true
striated muscle and nervous system tissues have negligible capacity to proliferate and in general do not undergo hyperplasia
true
Hyperplasia differs from neoplastic cellular proliferation in that it generally subsides if the stimulus is removed
true
metaplasia
one differentiated cell type (epithelial or mesenchymal) is replaced by another cell type
suqamous metaplasia is a reparative response to
chronic inflammation (mastitis)
hormonal imbalance
vitamin A deficiency
trauma
amyloid
complex protein that accumulates within cells, complex homogeneous pink material
calcification
abnormal accumulation of calcium salts in soft tissue
gout
in bird and reptiles assoc. with renal disease due to low excretion of urates
cholesterol crystals
areas of previous hemorrhage or inflammation
inclusions
viral diseases
intranuclear/intracytoplasmic
early, almost universal sign of injury
cell swelling
will often look cloudy and pale
hydrophobic degeneration
cell swelling results from
loss of control of ions/water with net uptake of watewr
loss of energy control/production
not incompatible with life of the cell depending on severity and is often mild and rapidly reversible
also occurs in lethal injury
Fatty change
accumulation of neutral fats in a cell, specialized in cells that metabolize lipids(hepatocyte,myocardial cells)
pathogenesis of fatty change
overload = diabetes/ anorexia
injury to cells = toxins/anoxia
deficiencies = methionine/choline
lipidosis = normal in milk diet/fatty meals
hepatic lipidosis
increased mobilization of body fat stores
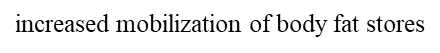
glycogen accumulation is due to
severe prolonged hyperglycemia
presence of high levels of glucocorticoids
lysosomal storage disease
pathogenesis for glycogen accumulation
prolonged, severe hyperglicemia = diabetes
increased corticosteroids
cushing’s syndrome = adrenal glands
iatrogenic
enzyme diseases
lipofuscin is a pignment that accumulates in
long lived postmitotic cells = neurons/cardiac myocytes
hyaline cartilage
intracellular or extracellular proteinaceous substances that take up eosin dye homogeneously
examples of hyaline changes
protein casts in renal tubules
plasma in blood vessel walls
thickened basement membranes
acute respiratory distress syndrome
fibrin thrombi
amyloidosis
protein misfolding disorders
you’ll see pale, waxy and translucent
gross appearance of lipidosis
yellow, priable, greasy organ
congo red stain highlights
amyloid deposition due to misfolding proteins
primary amyloidosis
the primary site affected by the deposition
secondary/systemic amyloidosis/serum amyloid AA
produced mainly by hepatocytes cleaved into fragments deposited as amyloid fibrils in kidneys, liver, spleen
due to chronic inflammation
dyscracias or neoplastic proliferations of plasma cells (B cells) produce
immunoglobulin light chains derived from plasma cells AL amyloid
some localized forms in nose of horses
calcification
abnormal deposition of calcium salts
dystrophic calcification
in areas of necrosis
calcification of skin = calcinosis circumscripta calcinosis cutis
vitamin E or selenium deficiency : my
metastatic calcification
HYPERCALCEMIA
chronic kidney disease
renal failure
toxicosis with vit.D
increased secretion of PTH
resorption of bone tissue
in calcification, renal failure will cause calcium deposition in
lungs, pleura and endocardium
calcinosis circumscripta
calcium deposition usually at bony prominences (dermis or subcutis)
calcinosis cutis
seen mainl
gout has not been reported in
domestic mammals
gout pathogenesis
kidney failure
disturbance of purine meatbolism
high protein diets
2 forms of gout
visceral (common) and articular (rare)
cholesterol crystals
in areas of previous hemorrhage, necrosis or inflammation, chronic process
viral inclusion bodies
certain viral diseases
intranuclear or cytoplasmic or both
intranuclear viral inclusion
DNA viruses
herpesvirus
adenovirus
parvovirus
intracytoplasmic inclusions
RNA viruses
rabies
canine distemper
irreversible cell injury
transition between living and dead cell
morphologic hallmarks of irreversible cell injury
severe mitochondrial swelling
large flocculent densities in mitochondrial matrix
increased loss of proteins, enzymes, co-enzymes
greatly increased membrane permeability
causes of cell damage
hipoxia
ischemia
hipoxia
decrease in oxygen to affectes tissues
ischemia
loss of blood flow to affected tissues
causes for hypoxia
decreased blood oxygen : pulmonary / non-pulmonary
decreased blood flow : hypovolemia, vasoconstriction, cardiogenic, shock, substrates for anaerobic metabolism delivered
glycolytic pathway
causes for ischemia
hypovolemia
infarction
vasoconstriction
shock
loss of oxygen + loss of substrates
mechanisms of cell injury
interference with substrates or enzymes:
glycolisis
citric acid cycle
oxidative phosphorylation
Produce enzymes or molecules that degrade cell components
phospholipases
free radicals
list 3 pathogenesis of glycogen accumulation
prolonged severe hyperglycemia can cause
glycogen accumulation
which of the following calcification is seen in areas of necrosis
which of the following causes toxicosis calcification
causes of metastatic calcification