Molecular Geometry
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
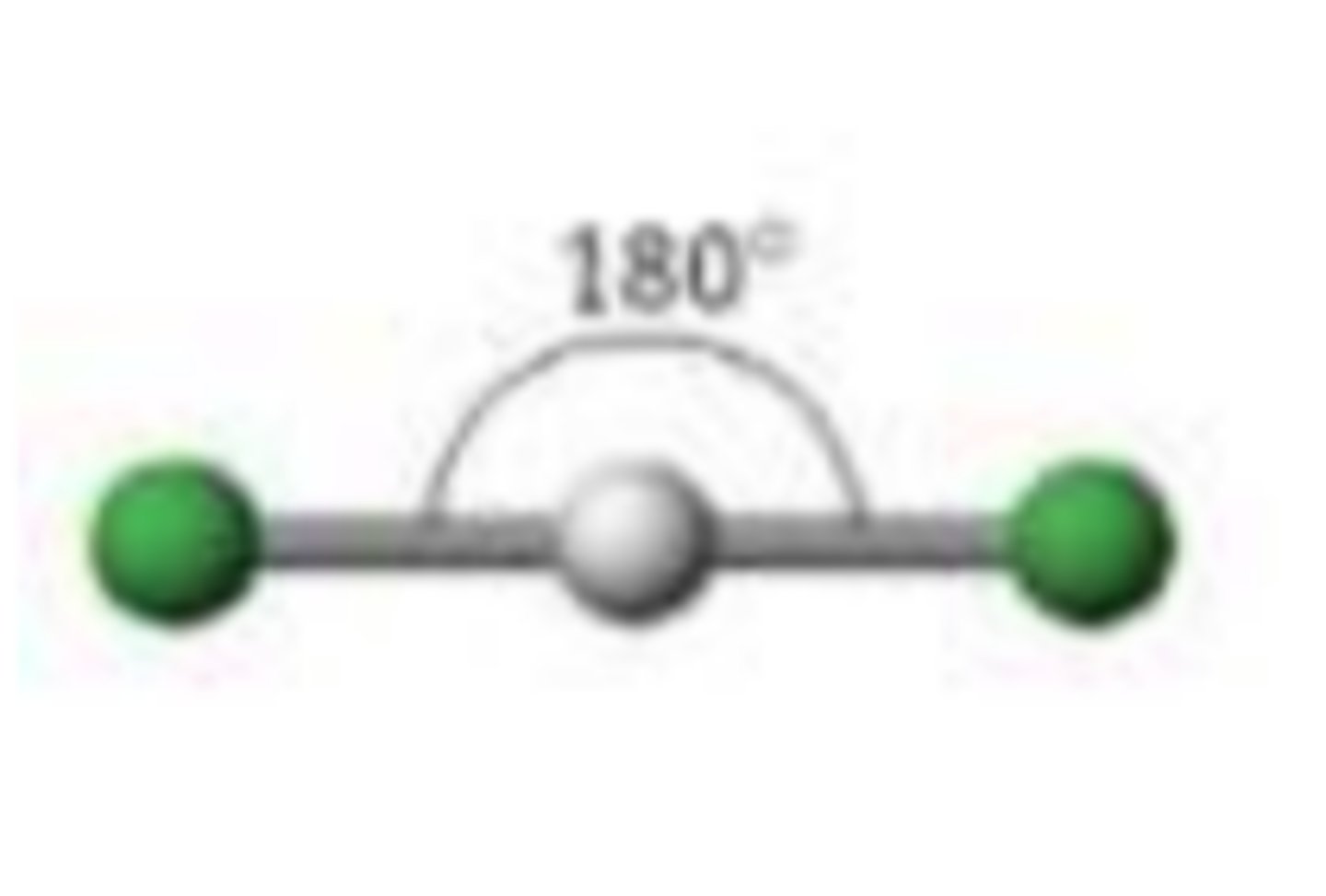
Linear (2 regions)
Two regions of electron density, two bonding pairs.
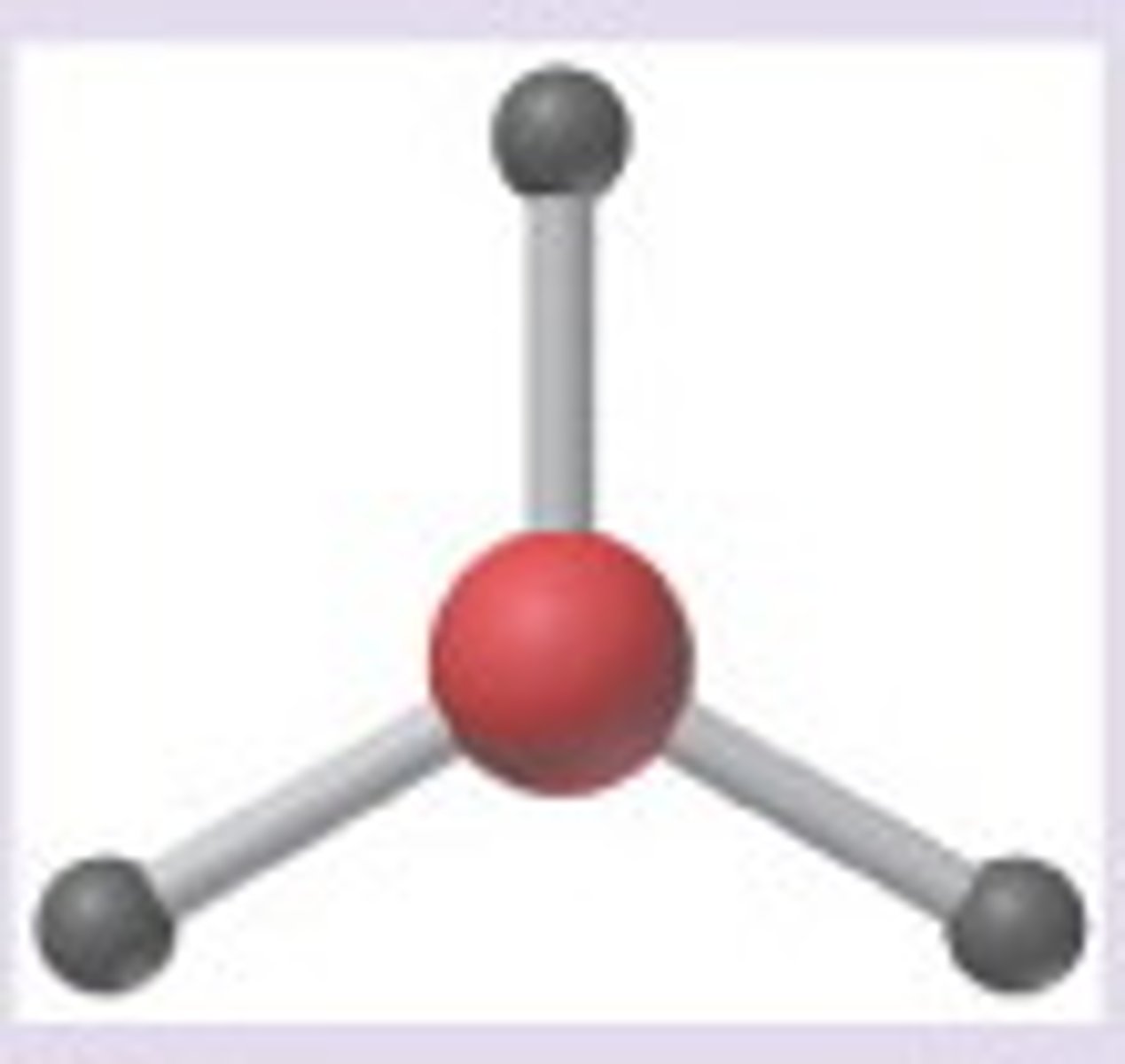
Trigonal planar
Three regions of electron density, three bonding pairs.
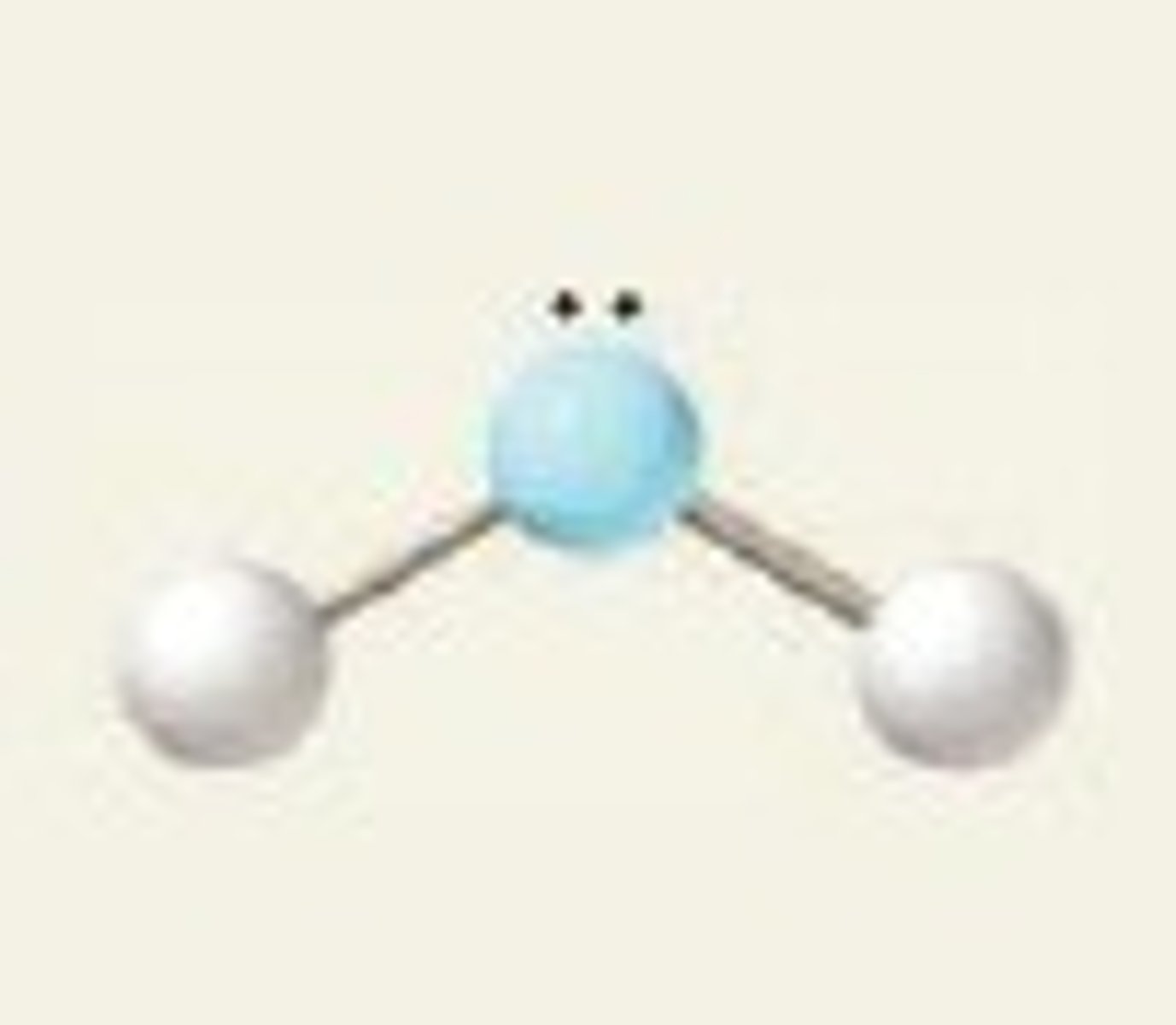
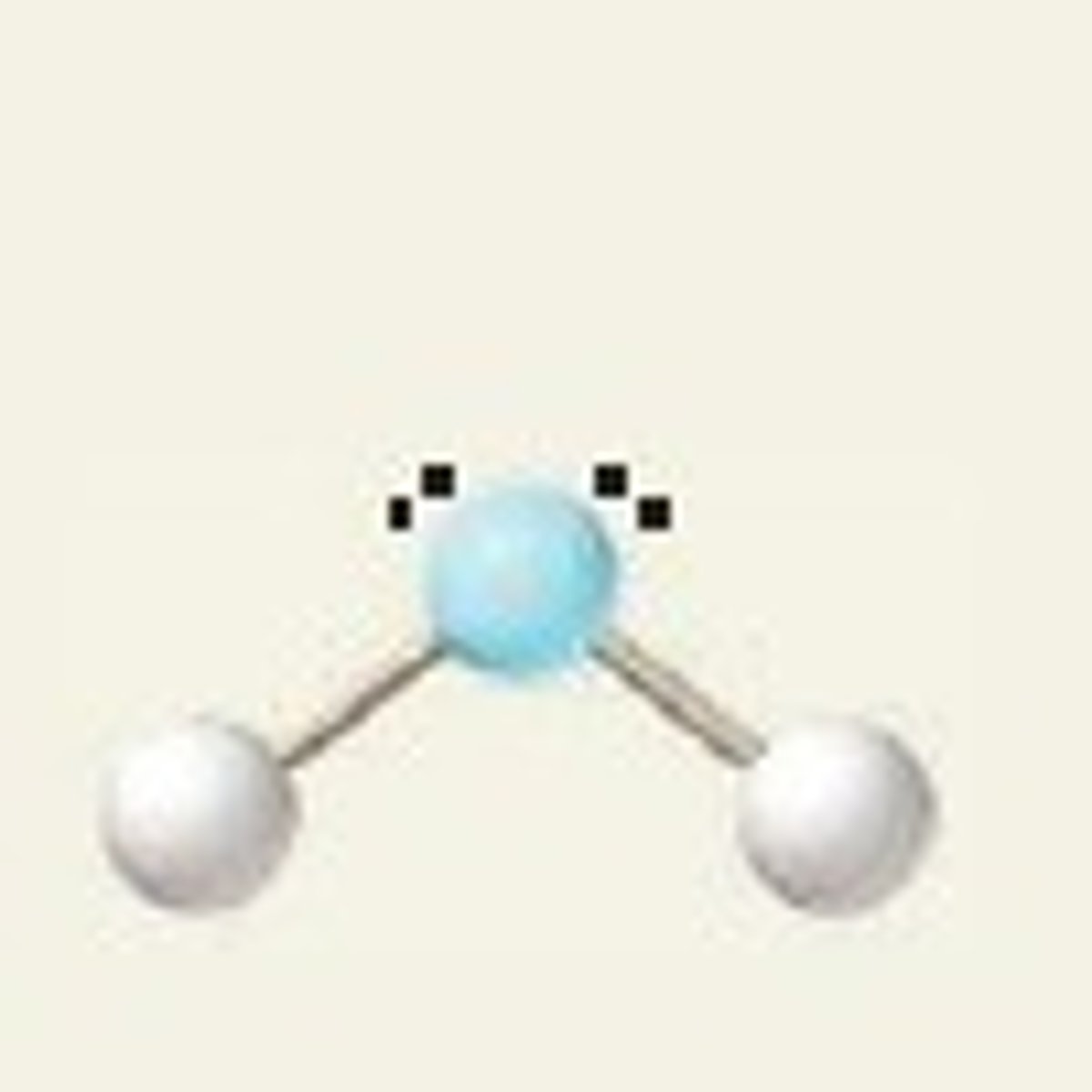
Bent (3 regions)
Three regions of electron density, two bonding pairs and one lone pair.
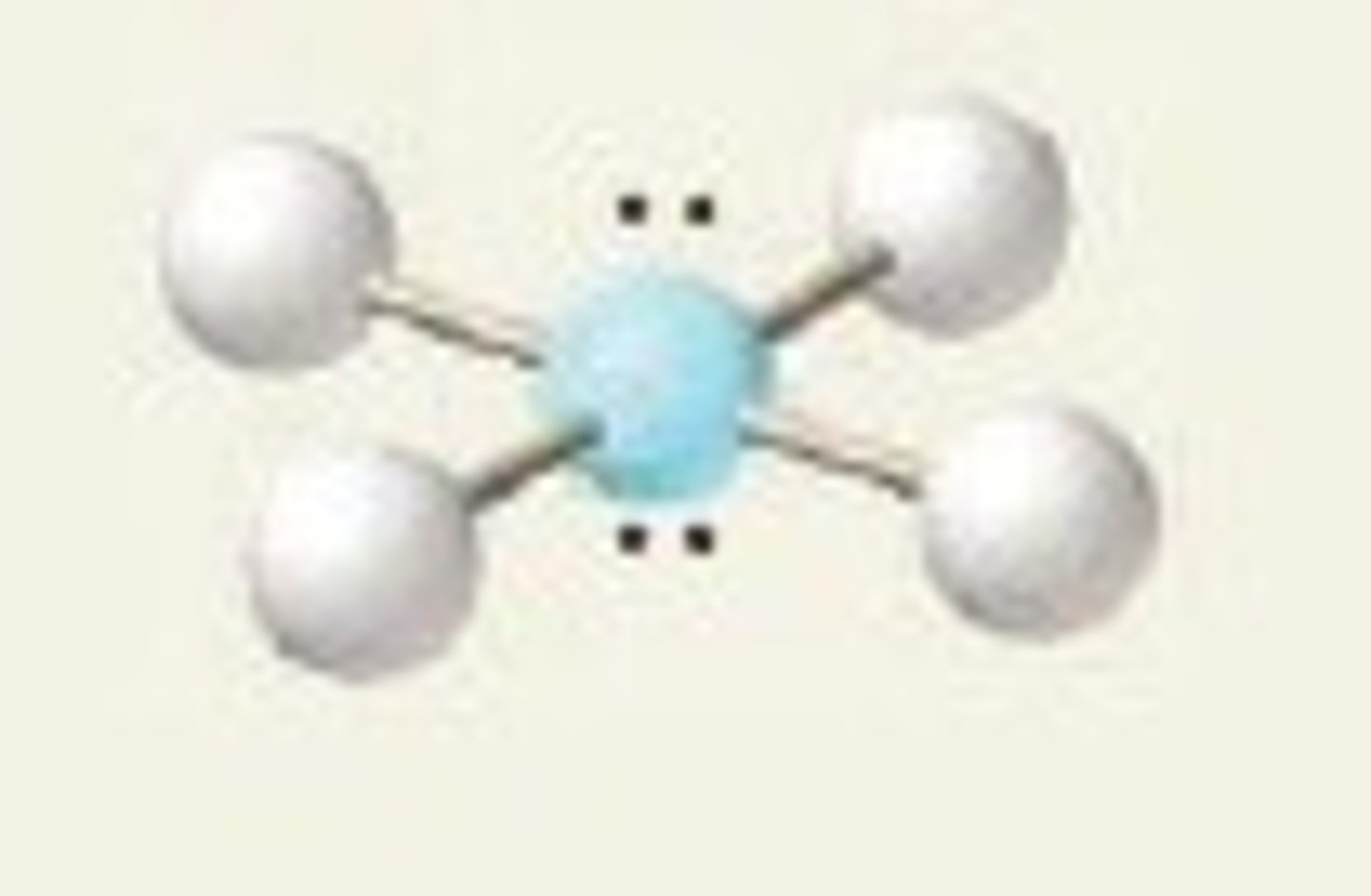
Tetrahedral
Four regions of electron density, four bonding pairs.
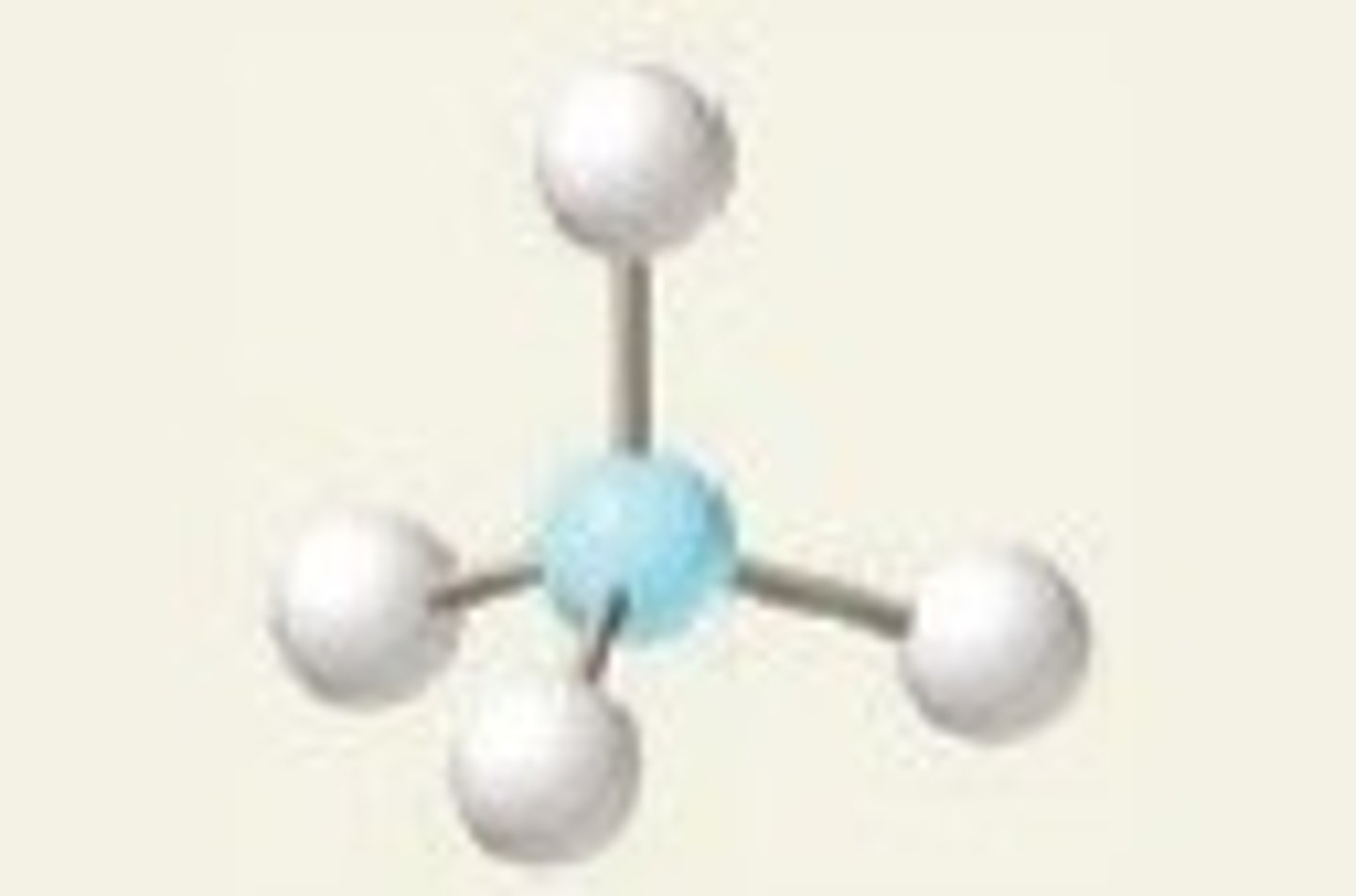
Trigonal pyramidal
Four regions of electron density, three bonding pairs and one lone pair.
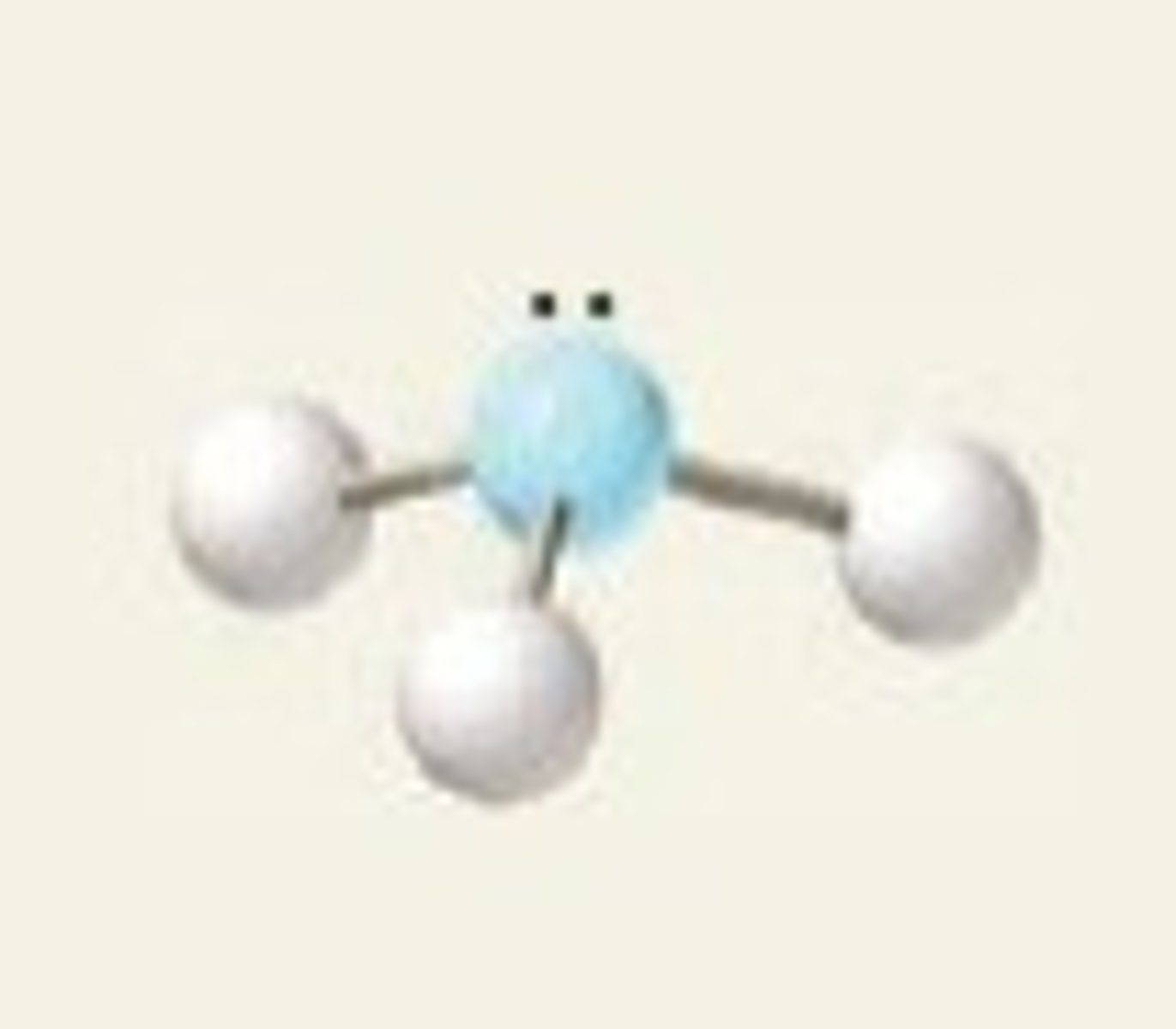
Bent (4 regions)
Four regions of electron density, two bonding pairs and two lone pairs.
Trigonal bipyramidal
Five regions of electron density, five bonding pairs.
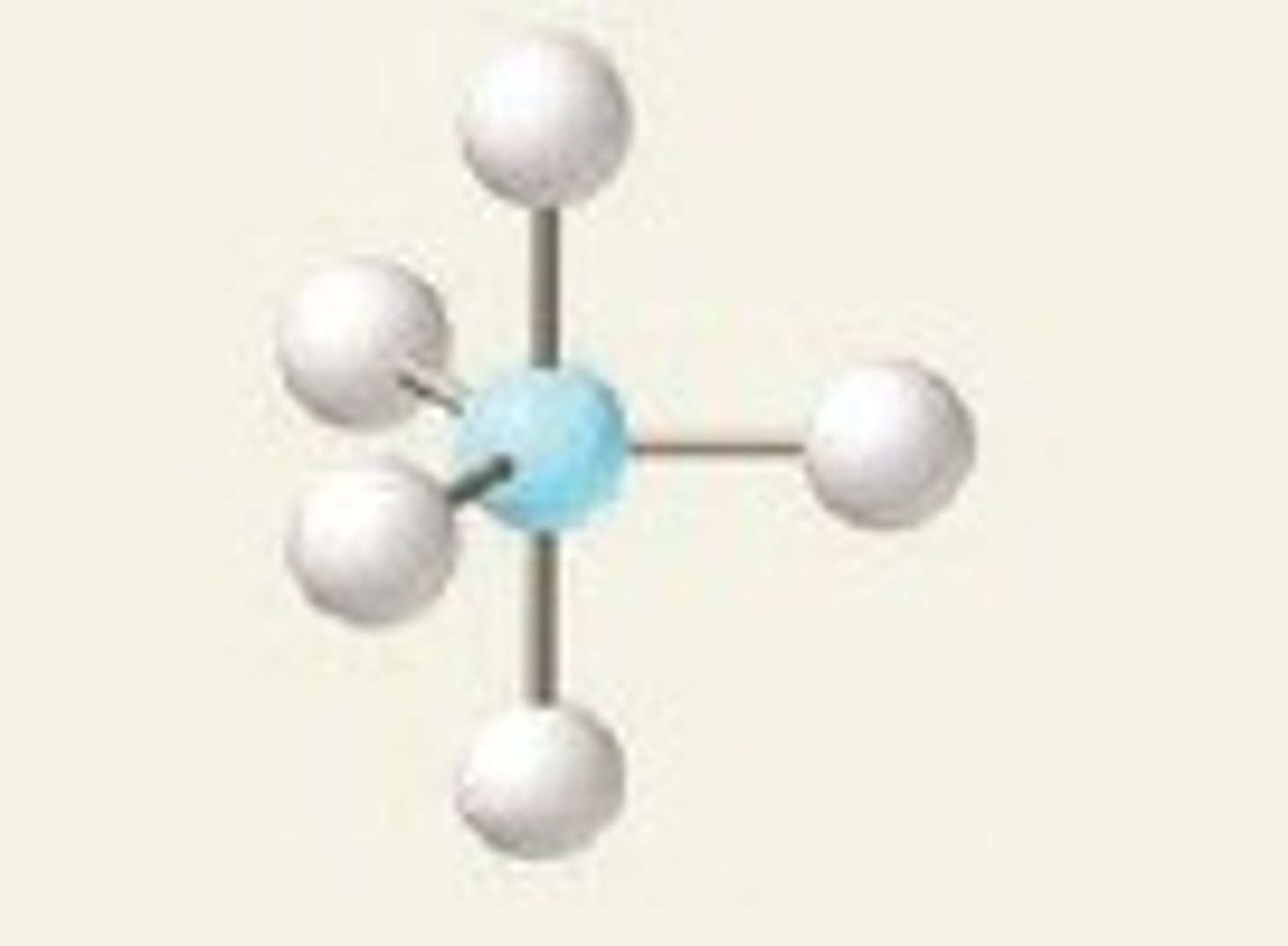
See-saw
Five regions of electron density, four bonding pairs and one lone pair (in equatorial position).
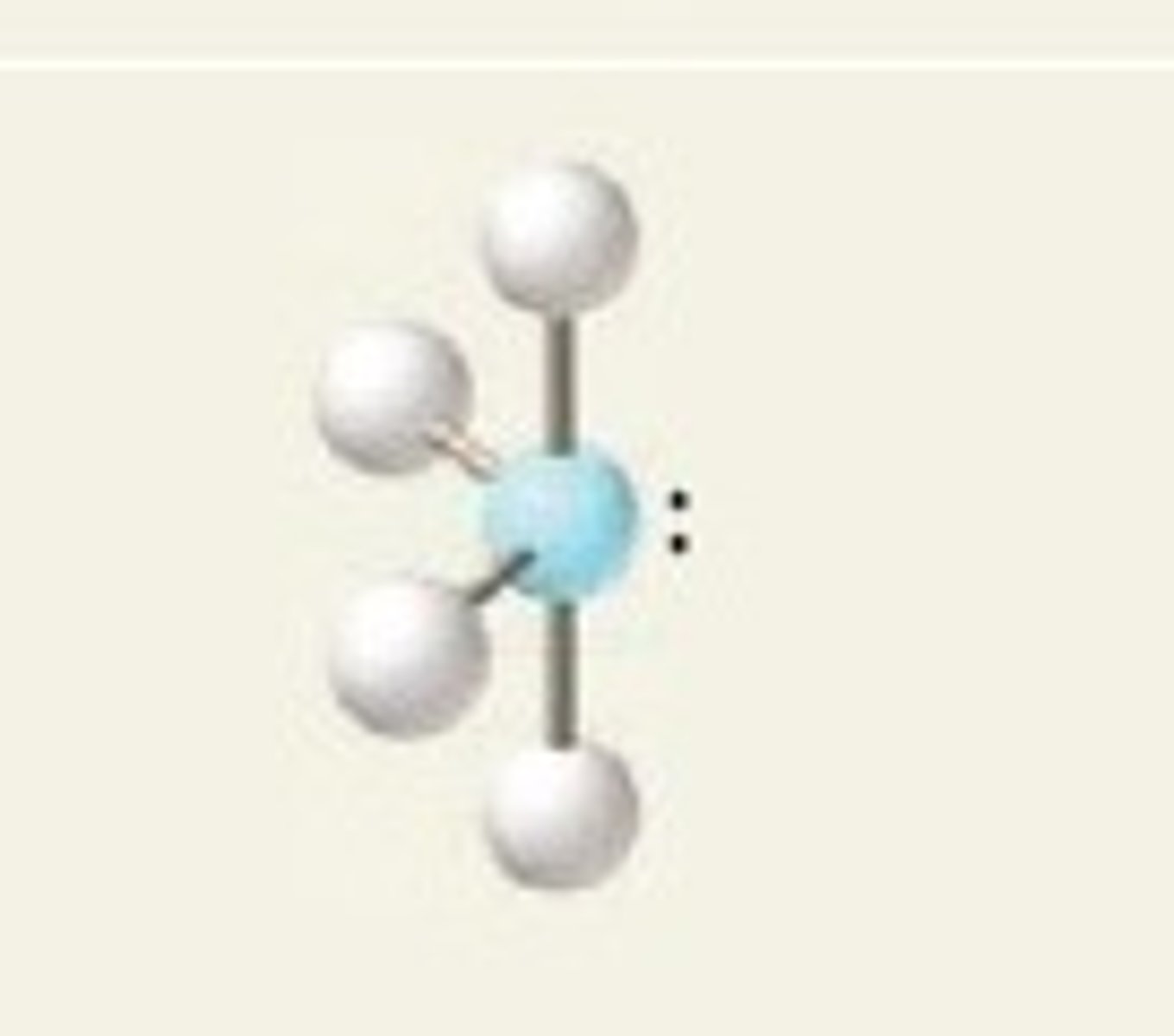
T-shaped (5 regions)
Five regions of electron density, three bonding pairs and two lone pairs (in equatorial positions).
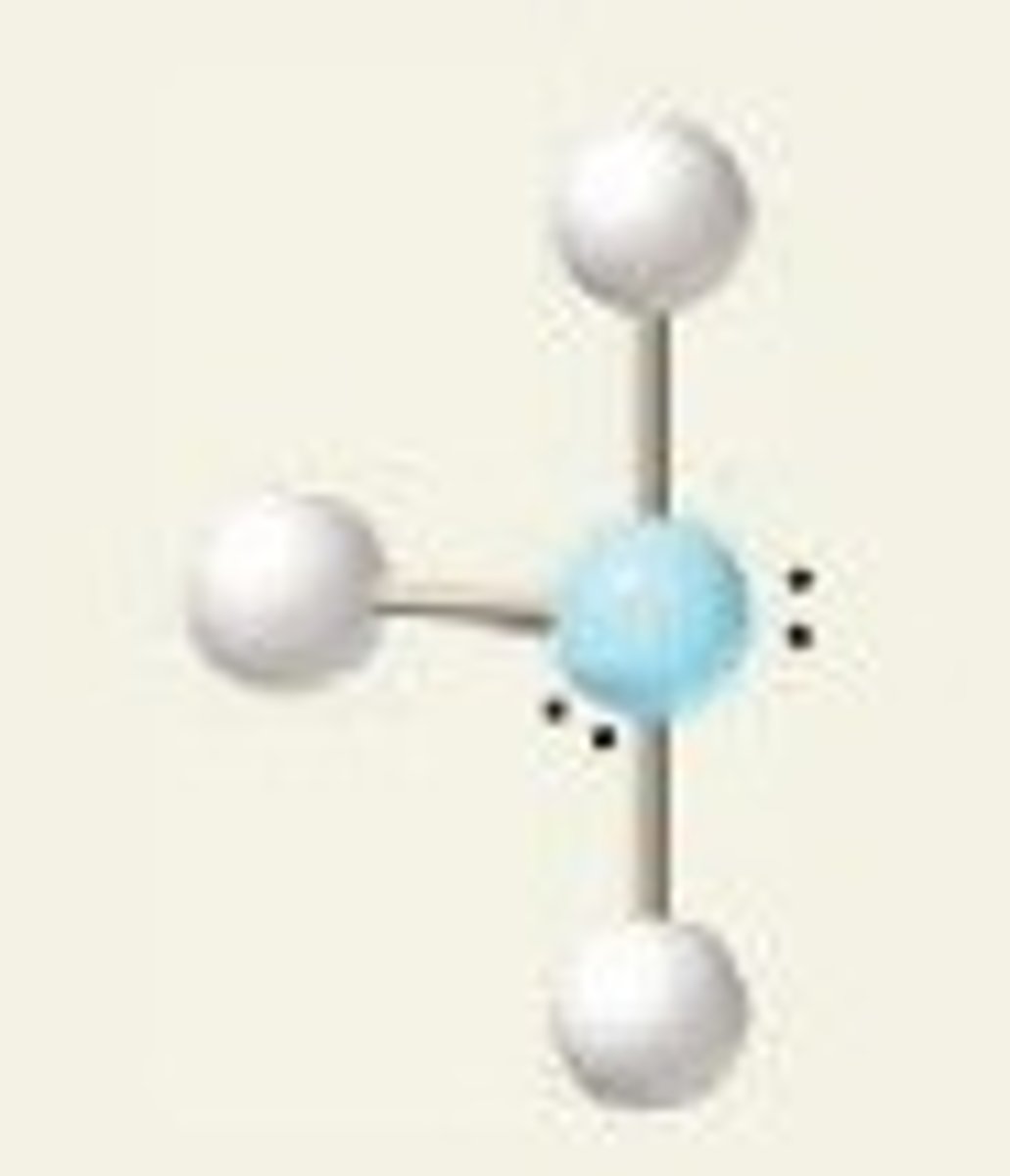
Linear (5 regions)
Five regions of electron density, two bonding pairs and three lone pairs.
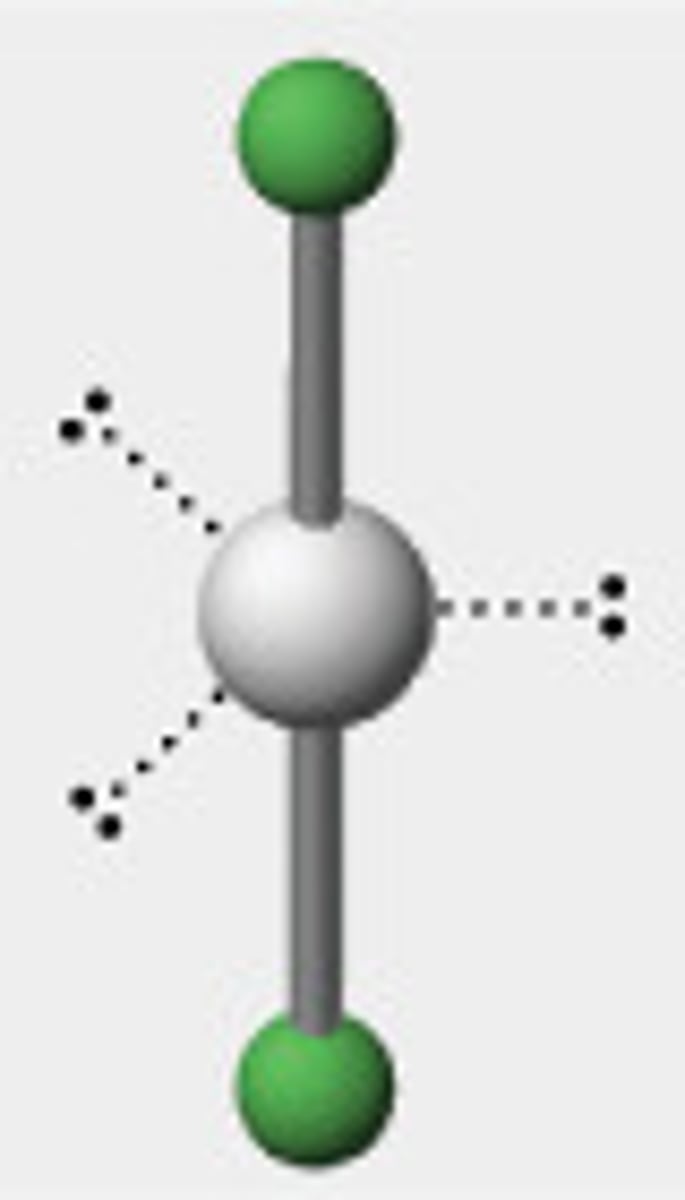
Octahedral
Six regions of electron density, six bonding pairs.
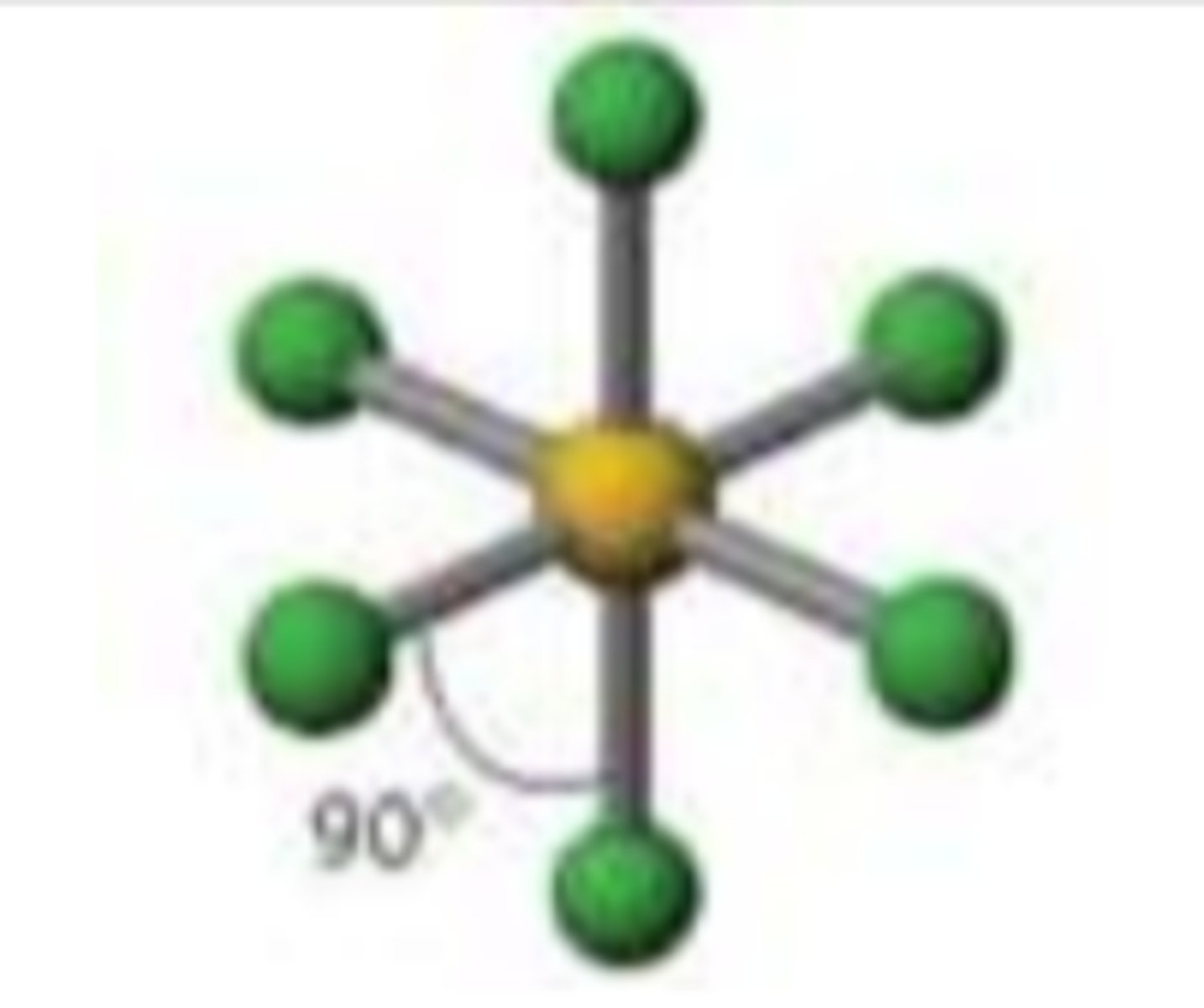
Square pyramidal
Six regions of electron density, five bonding pairs and one lone pair. (in axial position).
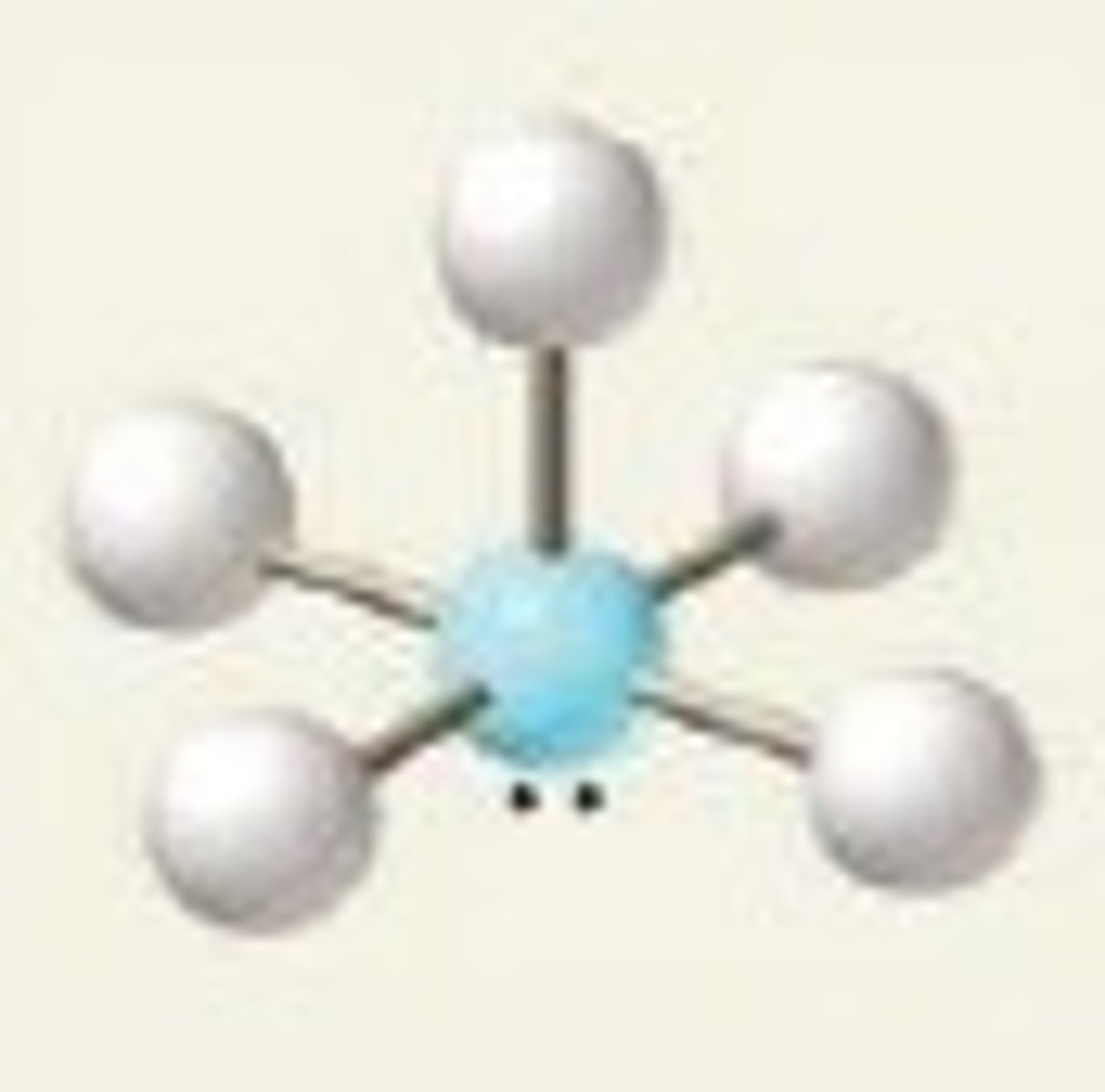
Square planar
Six regions of electron density, four bonding pairs and two lone pairs (both in axial position).