Chem Test 1 (Pure substances, solids, mixtures, etc.)
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Pure Substances
Single kind of matter that can’t be broken down (Gold, Copper Wire, Sugar, Salt, Hydrogen Gas, Diamonds, etc).
Element
Made from one kind of atom; cannot be broken down
Compound
Substance made from more than one element; can be broken down into elements (pure substances).
Matter
Anything that has mass and takes up volume.
Mixtures
When elements are combined physically; can be separated by physical means.
Homogenous Mixtures
Mixtures that have uniform composition; appear to be one substance but are actually multiple.
Heterogenous Mixtures
Mixtures that don’t have a uniform composition; appear to be multiple substances
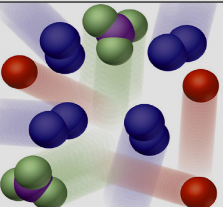
¿Que está?
Mixture
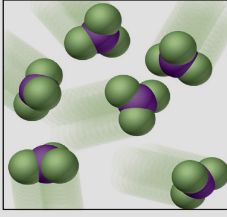
¿Que está?
Compound
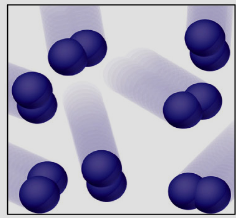
¿Que está?
Element
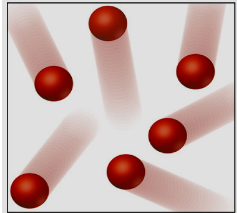
¿Que está?
Element
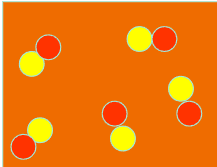
¿Que está?
2 different elements forming a compound
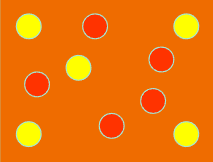
¿Que está?
2 different elements forming a mixture
Decant
Separates liquids from solid; solids settle at bottom of beaker, and you separate the two by stirring the liquid and then pouring it out of the beaker.
Filtration
Separates solids from fluids; passes a mixture through a porous material that lets the fluid pass through but not the solid.
Evaporation
Separates liquids from solid in a solution; allows the liquid to turn into a vapor, leaving the soluble solid behind.
Crystallization
Separates pure solid particles from a liquid solution creating the dissolved substance. As liquid substance evaporates, the atoms from the dissolved solid come out of the solution and solidifies.
Non-magnetic metals
Gold, Silver, and Aluminum
Sifting
Separates a mixture of different substances based on their size.
Extraction
Separates an insoluble solid from a soluble solid by adding a liquid that dissolves one part of the mixture, then pour it through a filter to separate the undissolved part, allowing the dissolved part to pass through.
Chromatography
Separates dissolved substances in a solution from each other by a special paper.
Centrifuge
Separates materials in a solution by density through a machine that rotates containers of liquids at high speeds.
Distillation
The process of separating a liquid homogenous mixture; the separation of liquids based on their boiling points.
Magnetic metals
Iron, Nickel, and Cobalt
Insoluble solid
Solids that can’t dissolve in a liquid.
Soluble solid
Solids that can dissolve in a liquid.