wack ahh dynamics
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
isobaric
constant pressure
isothermal
constant temperature
isochoric
constant volume
adiabatic
no heat exchange, steeper than isothermal
when work is done on system means…
negative sign
if you lose heat in a system
negative sign
internal energy increases
positive sign
in the first law equation work_____ heat
can not be more than
1st law equation for isothermal

1st law equation for isochoric
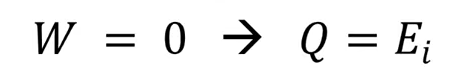
1st law equation adiabatic
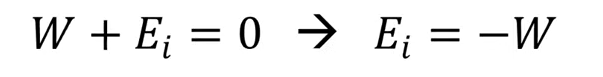
irreverseable process
number of states increase
reversable process
number of states has not changed
temperature in _____
kelvin
in Linear thermal expansion temp is
Celsius or kelvin
for two materials with different values of a, which way will kt bend when heated
towards the one with a lesser value of a
1 kcal
1000 cal = 4186 J
specific heat unit
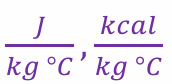
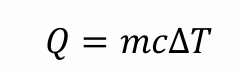
what’s the temp unit for this equation
Celsius or kelvin
Triple Point
all three states of mater exist at this point
order of phase diagram left to right
solid, liquid, gas
Specific heat is ONLY used when the ___________
temperature changes
latent heat
heat for phase changes
latent heat unit
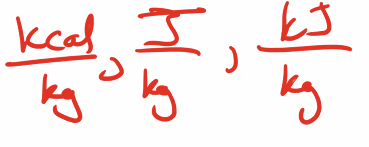
Gas units
volume: m3 temp: k pressure: Pa
N
Number of molecules
n
Number of moles
(a) unit (in thermal expansion equation)
1/oC