Extraction and uses of metals - IGCSE Edexcel Chemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Metals are extracted from.........
............metal ores from the Earth's crust
Unreactive elements like Ag and Au .......
....... are found uncombined
Haematite
A common iron ore
Bauxite
A common aluminium ore
Malachite
A common copper ore
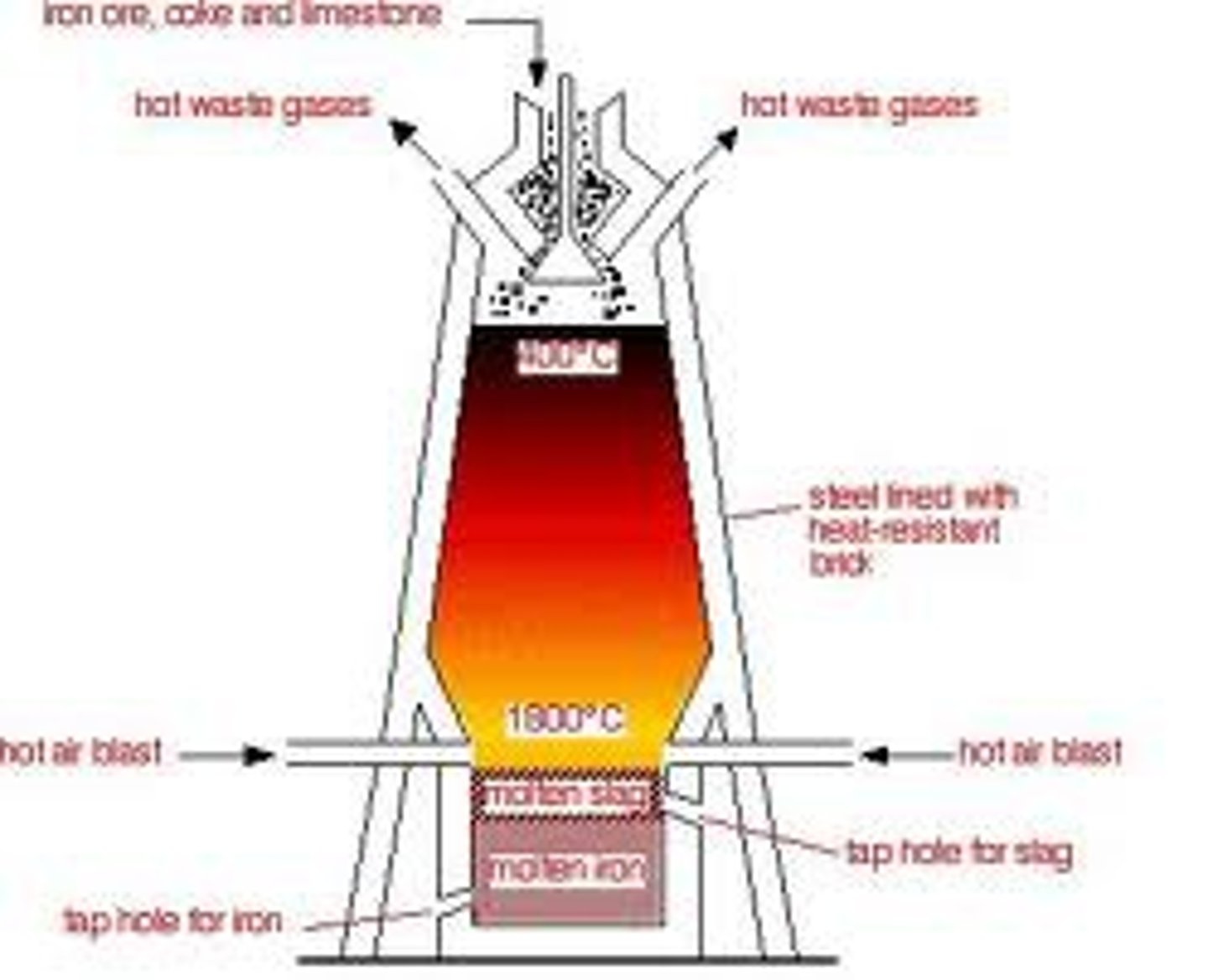
Metals above carbon are extracted by.................
...............Electrolysis. e.g. iron in electrolysis
Metals below carbon are extracted by.............
.............carbon. e.g. iron in the blast furnace
Uses of aluminium
-Airplane manufacture (low density)
-Power cables (low density, good conductor)
-Cooking Pans(resistance to corrosion)

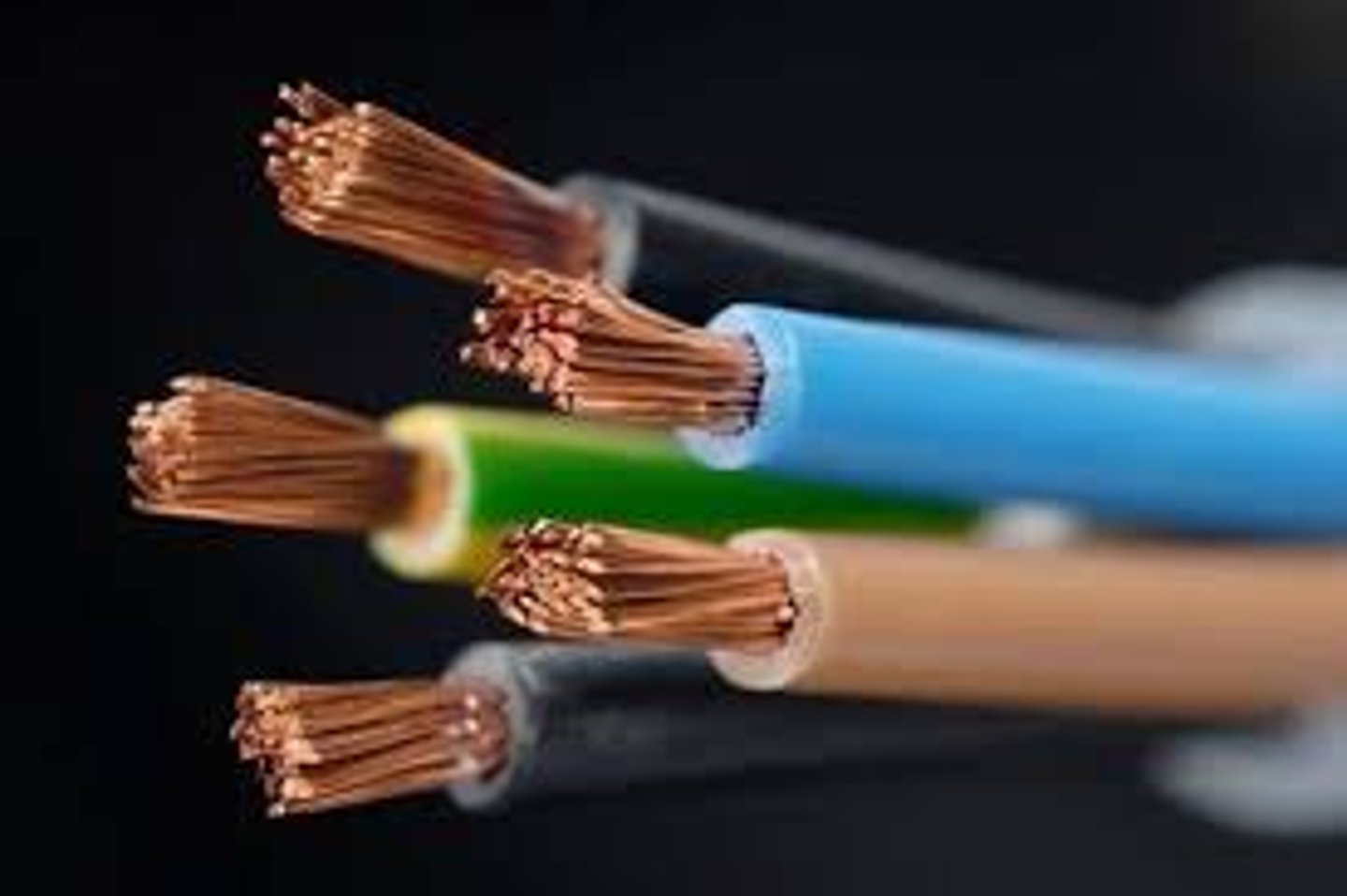
Uses of copper
Electrical wiring and plumbing
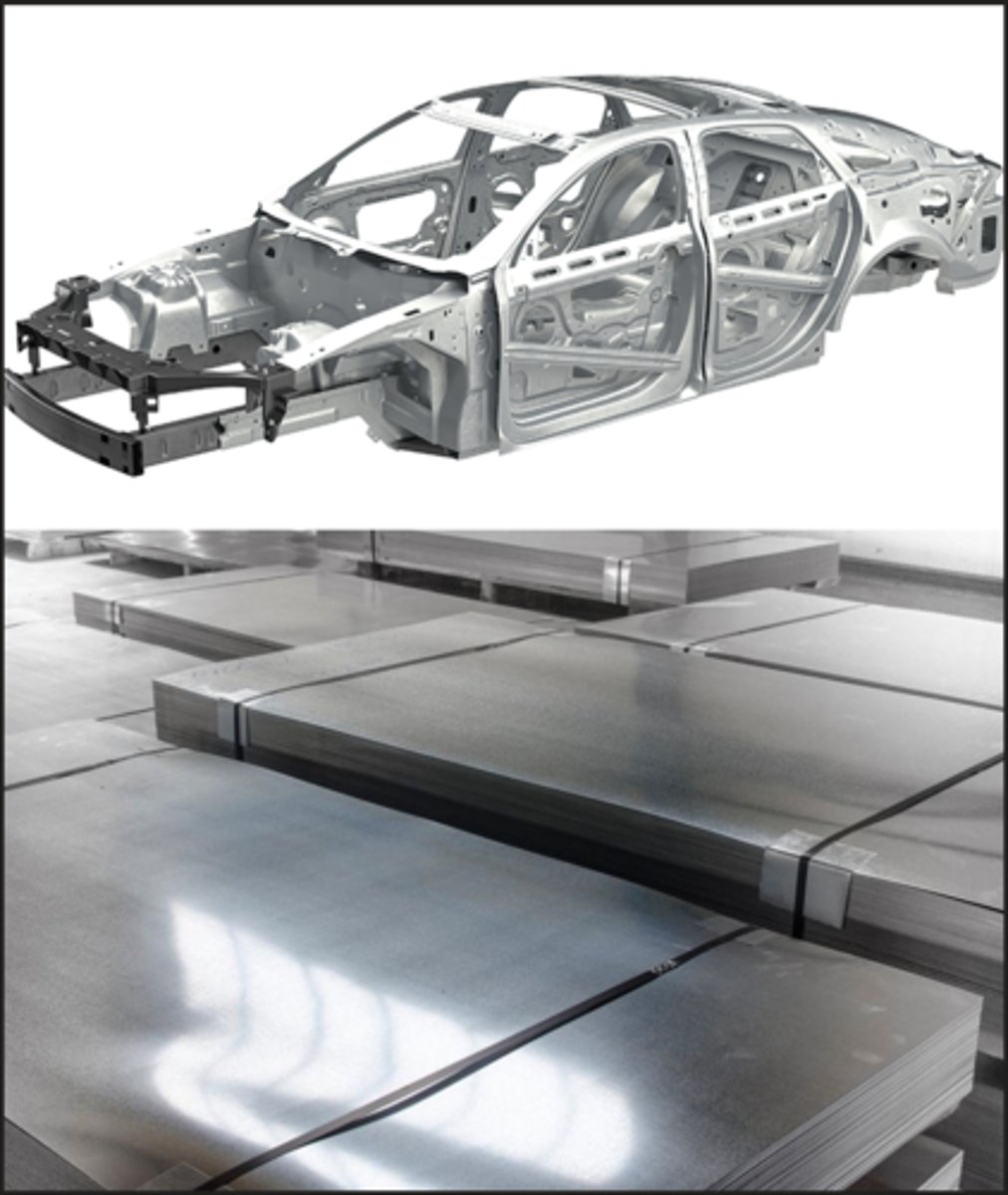
Uses of mild steel (low-carbon steel)
Car bodies and machinery
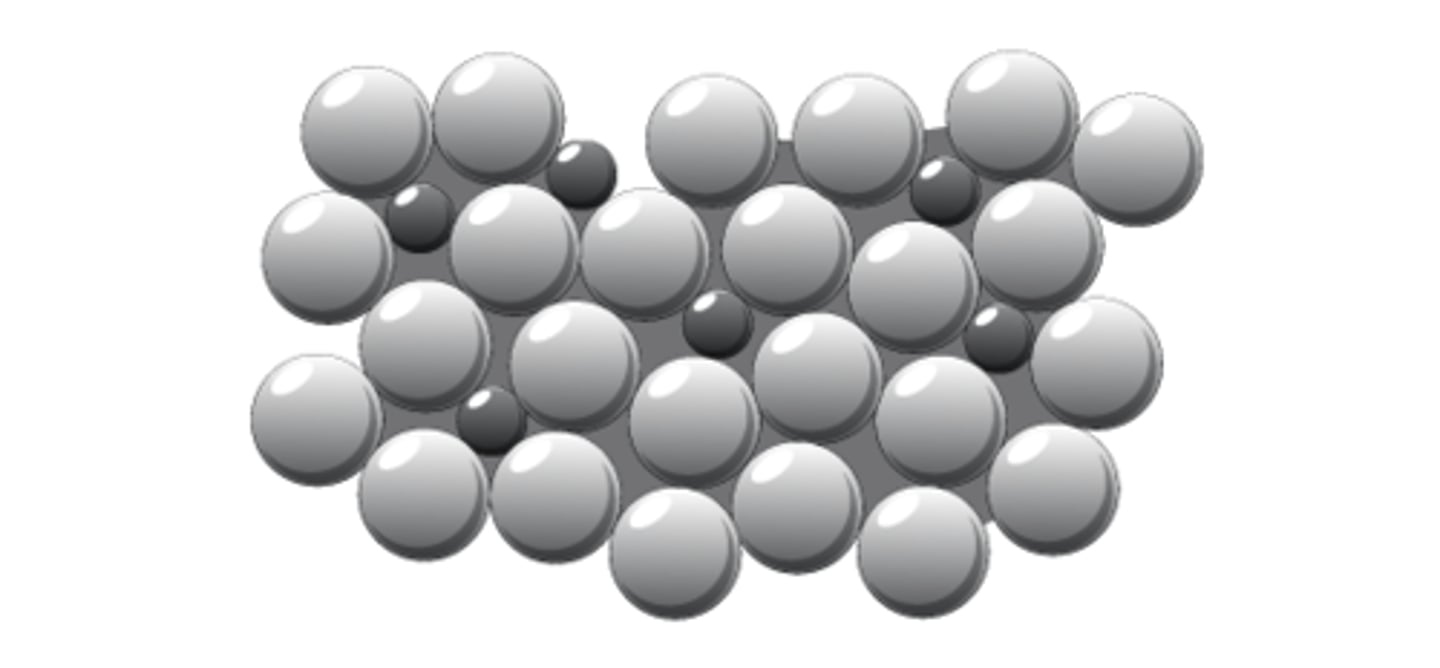
Alloys
mixtures of two or more metals e.g. brass, bronze.
Uses of high carbon steel
Cutting tools, ball bearings

Uses of stainless steel
cutlery,kitchen sinks, pots and pans, surgical instruments

Why are alloys harder?
They have different sized atoms which prevent the layers from sliding over each other
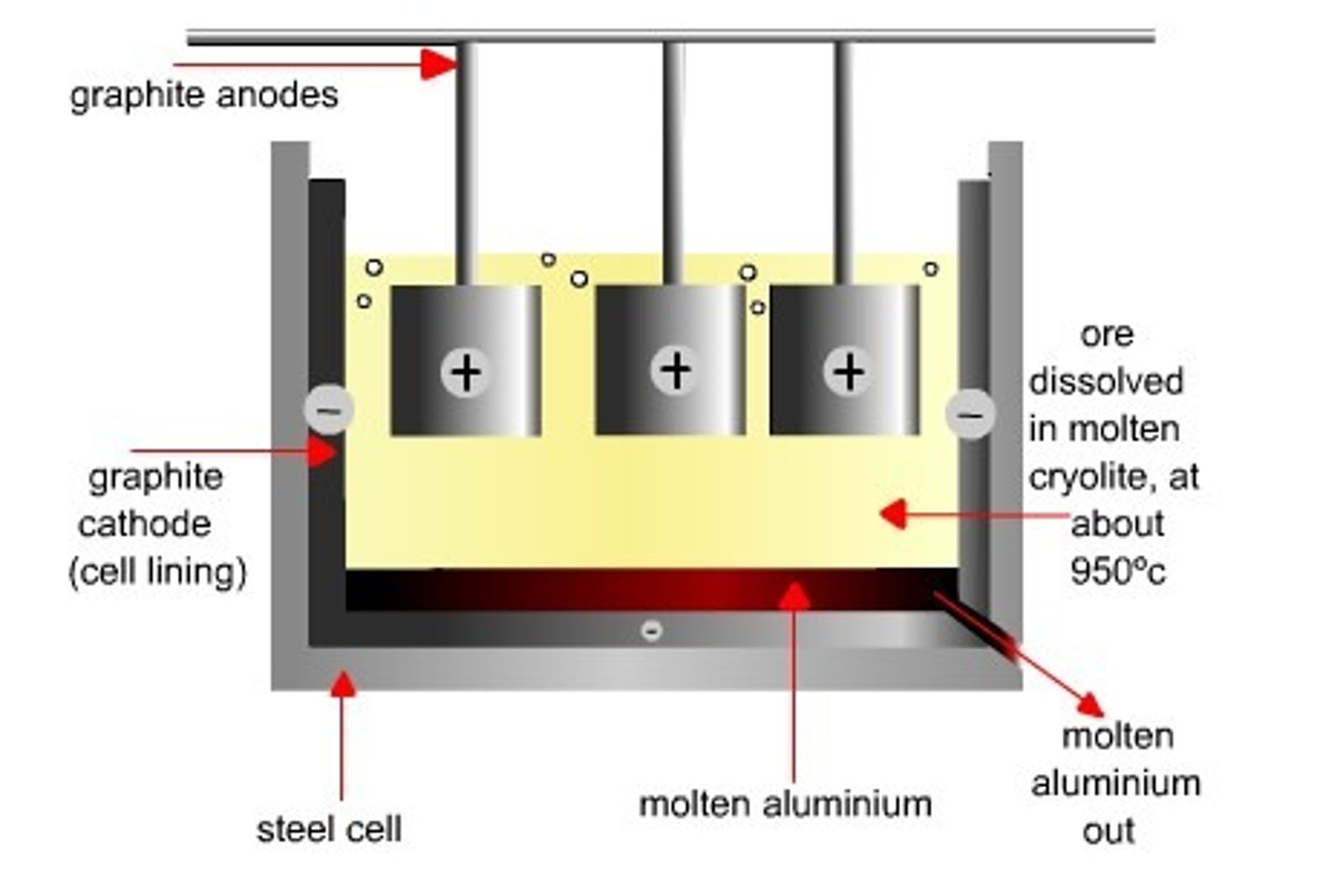
Electrolysis of aluminium
Mainly found in compounds (bauxite - Al2O3)
Aluminium Oxide is disolved in MOLTEN CRYOLITE
Aluminium has melting point of 2000°C - Cryolite lowers the operating temperature at which the reaction occurs to 1000°C
Blast furnace
The huge reaction vessels used in industry to extract iron from its ore