PCOL3022 Lecture 8: Neuropeptides
0.0(0)
Card Sorting
1/25
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
1
New cards
What are Neuropeptides?
- A small protein or peptide that acts as a neurotransmitter in the nervous system
- Found in the CNS and PNS
- Some can also act as hormones (e.g. oxytocin, vasopressin)
- More than 100 identified so far
- Many have historical names linked to their first identified function (e.g. somatostatin reduces release of growth hormone)
- Found in the CNS and PNS
- Some can also act as hormones (e.g. oxytocin, vasopressin)
- More than 100 identified so far
- Many have historical names linked to their first identified function (e.g. somatostatin reduces release of growth hormone)
2
New cards
What is the structure of neuropeptides?
- Linear polymer made of amino acids joined by peptide bonds
3
New cards
How do we determine the effects of neuropeptides?
1. Using selective agonists and antagonist
= Defines cellular and behavioural effects
= Antagonists can be difficult due to effects like poor antagonism, inverse agonism (stops action of the receptor, not the agonist)
2. Knock-out animals
= Compensation was a major issue
= Conditional knock-outs largely avoid this
3. Peptidase inhibitors
= These will increase the effects of endogenous NPs
= Defines cellular and behavioural effects
= Antagonists can be difficult due to effects like poor antagonism, inverse agonism (stops action of the receptor, not the agonist)
2. Knock-out animals
= Compensation was a major issue
= Conditional knock-outs largely avoid this
3. Peptidase inhibitors
= These will increase the effects of endogenous NPs
4
New cards
What are endogenous opioids involved in?
- Mood
- Pain perception
- Drug addiction
- Decision making
- Fear response
- Stress response
- Attachment formation
- Gastrointestinal (GI) function
- Pain perception
- Drug addiction
- Decision making
- Fear response
- Stress response
- Attachment formation
- Gastrointestinal (GI) function
5
New cards
What do endogenous opioids do?
1. Agonists
= They define what activation of the receptor can do
= Cause euphoria, pain relief, constipation, respiratory depression
= Tells you what system is important for but not what endogenous opioids are doing
2. Antagonists
= Shows effects at a behavioural level
= Stress-induced analgesia is negated by naloxone (opioid agonist)
3. Knock outs (either receptor or peptide)
= Opioid KO animals are highly anxious, fearful, have altered sexual activity and good palatability
4. CRISPR
= Knocks down peptides in particular areas for particular behaviors
5. Peptidase inhibitors
= Enkephalin-degrading enzyme inhibitors used
- Antinociceptive, antidepressant and anxiolytic effect in rodents
= They define what activation of the receptor can do
= Cause euphoria, pain relief, constipation, respiratory depression
= Tells you what system is important for but not what endogenous opioids are doing
2. Antagonists
= Shows effects at a behavioural level
= Stress-induced analgesia is negated by naloxone (opioid agonist)
3. Knock outs (either receptor or peptide)
= Opioid KO animals are highly anxious, fearful, have altered sexual activity and good palatability
4. CRISPR
= Knocks down peptides in particular areas for particular behaviors
5. Peptidase inhibitors
= Enkephalin-degrading enzyme inhibitors used
- Antinociceptive, antidepressant and anxiolytic effect in rodents
6
New cards
How do endogenous opioids act to produce these effects?
- Lots of effects but few responses
- High-frequency stimulation suggests a role in learning events
- High-frequency stimulation suggests a role in learning events
7
New cards
Steps in Neuropeptide Synthesis
- Many steps regulate this process, meaning that one gene can produce multiple peptides
Steps:
1. mRNA transcription
2. Mature mRNA translated into inactive prepropeptide at ribosome
3. N-terminal cleavage by peptidase gives propeptide
4. Propeptide packaged into DCV (dense core vesicles) in trans-Golgi network
5. Peptide undergoes post-translation processing occur in this DCV
Steps:
1. mRNA transcription
2. Mature mRNA translated into inactive prepropeptide at ribosome
3. N-terminal cleavage by peptidase gives propeptide
4. Propeptide packaged into DCV (dense core vesicles) in trans-Golgi network
5. Peptide undergoes post-translation processing occur in this DCV
8
New cards
How is neuropeptide synthesis different from regular NT synthesis?
- Neuropeptide synthesis requires transcription and translation
- Regular NT synthesis is a simple production with vesicles transported + released at classical synapse
- Regular NT synthesis is a simple production with vesicles transported + released at classical synapse
9
New cards
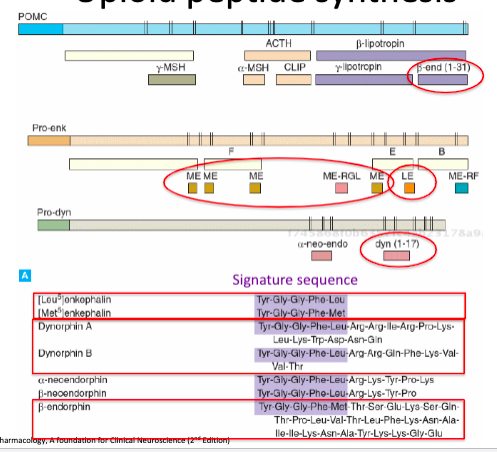
Opioid Synthesis
1. POMC
- Long propeptide
- Multiple peptides derived from it including B-endorphin
2. Pro-enk
- Multiple Leu- and Met-enkephalins derived from single propeptide
- Each consists only of the opioid signature sequence, Tyr-Gly-Gly-Phe-Leu/Meet, required for OR activation
- Enkephalins thought to be responsible for most peptidase inhibitor effects as their small size means they are susceptible to degradation
3. Pro-dyn
- Dynorphins A and B derived
- Long propeptide
- Multiple peptides derived from it including B-endorphin
2. Pro-enk
- Multiple Leu- and Met-enkephalins derived from single propeptide
- Each consists only of the opioid signature sequence, Tyr-Gly-Gly-Phe-Leu/Meet, required for OR activation
- Enkephalins thought to be responsible for most peptidase inhibitor effects as their small size means they are susceptible to degradation
3. Pro-dyn
- Dynorphins A and B derived
10
New cards
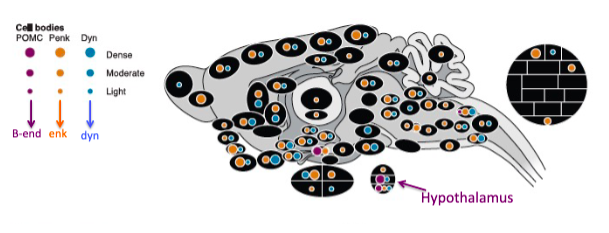
Opioid Peptide Expression
1. POMC cell bodies concentrated in hypothalamus but project more widely
2. Penk and Dyn more widespread (both cell bodies and axons)
- POMC's location in hypothalamus suggests role in coordinated responses (i.e. only one region is activated but multiple peptides released)
2. Penk and Dyn more widespread (both cell bodies and axons)
- POMC's location in hypothalamus suggests role in coordinated responses (i.e. only one region is activated but multiple peptides released)
11
New cards
Storage and Release of Neuropeptides
- DVC contain 10,000 peptide molecules each
- SCNs (small clear vesicles) store monoamine NTs
- Some neurons contain many peptides/NPs (e.g. supraoptic neurons)
- Differences in Ca2+ sensor location and the sensitivity of release (lots of DCVs do not release contents in "normal" part of the terminal where [Ca2+] is high)
-Terminal or dendritic release may require multiple different proteins, can be non-synaptic
- Limited number in terminal - NPs are degraded instead of having a reuptake mechanism, meaning they can take hours to replenish (has led to hypothesis that NPs act in short bursts)
- Movement from cell body can be regulated by neuronal activity y
- SCNs (small clear vesicles) store monoamine NTs
- Some neurons contain many peptides/NPs (e.g. supraoptic neurons)
- Differences in Ca2+ sensor location and the sensitivity of release (lots of DCVs do not release contents in "normal" part of the terminal where [Ca2+] is high)
-Terminal or dendritic release may require multiple different proteins, can be non-synaptic
- Limited number in terminal - NPs are degraded instead of having a reuptake mechanism, meaning they can take hours to replenish (has led to hypothesis that NPs act in short bursts)
- Movement from cell body can be regulated by neuronal activity y
12
New cards
Cellular Actions
- They are mostly studied at hypothalamic hypophysis (here we see a large number of large DCVs)
- May not accurately reflect peptide release at typical synapses
- May not accurately reflect peptide release at typical synapses
13
New cards
Opioid peptide storage and release
- Amygdala: the DCV/SCV ratio is much smaller and the DCVs are smaller
- Stored in DCVs
- Terminal have DCVs and SCVs
- Hypophysis - high stimulation (slamming) to get them to release -- not necessarily the case in the rest of the brain
- Stored in DCVs
- Terminal have DCVs and SCVs
- Hypophysis - high stimulation (slamming) to get them to release -- not necessarily the case in the rest of the brain
14
New cards
What happens after opioid peptide release?
- NPs work on GPCRs
- Can spread much longer distances than NTs
- Most have a high affinity for their peptides (to be able to produce an effect -- high concentrations)
- Some NPs act at multiple receptors, some have multiple peptide agnoists
- Can be autoreceptors, post-synaptic, or extra-synaptic
- Receptors can be internalised (and signal)
- Can spread much longer distances than NTs
- Most have a high affinity for their peptides (to be able to produce an effect -- high concentrations)
- Some NPs act at multiple receptors, some have multiple peptide agnoists
- Can be autoreceptors, post-synaptic, or extra-synaptic
- Receptors can be internalised (and signal)
15
New cards
Opioid Receptors
- Opioid receptors are expressed throughout the brain
- Distribution in the brain does not match peptide release
- Distribution in the brain does not match peptide release
16
New cards
What do opioid peptides do once released?
- Different agonists have different affinities between mu (μ), delta (δ), and kappa (κ) receptors (affinities for all the ORs are much higher than for NTs)
- All ORs are Gi/o-coupled
- Actions include:
= Decreased NT release
= Decreased adenylyl cyclase action
= Decreased Ca2+ entry
= Increased K+ exit
- All ORs are Gi/o-coupled
- Actions include:
= Decreased NT release
= Decreased adenylyl cyclase action
= Decreased Ca2+ entry
= Increased K+ exit
17
New cards
What happens once they released?
- No reuptake -- need to synthesise (may be feedback)
- Broken down by extracellular peptidase (lower levels than Acheterase)
- Broken down by extracellular peptidase (lower levels than Acheterase)
18
New cards
Opioid peptide degradation
- Performed by several peptidases
- Enkephalins are most susceptible
= Inhibiting enkephalinase --> NT synaptic currents are reduced
= Conversely, adding a receptor antagonist (naloxene) causes increased current
- Enkephalins are most susceptible
= Inhibiting enkephalinase --> NT synaptic currents are reduced
= Conversely, adding a receptor antagonist (naloxene) causes increased current
19
New cards
Cellular effects of endogenous opioids
- If opioids are released with low stimulation --> actions are prevented by peptidases
- When released with moderate stimulation --> peptidases are overwhelmed, and we see post-synaptic activation of GIRKs
- Action at typical CNS synapses may differ to neurohypophysis
= NPs may have different roles in other parts of the CNS
- When released with moderate stimulation --> peptidases are overwhelmed, and we see post-synaptic activation of GIRKs
- Action at typical CNS synapses may differ to neurohypophysis
= NPs may have different roles in other parts of the CNS
20
New cards
Role of Neuropeptides
- Another layer of inhibition and excitation control
- Act more like 5-HT, NAd than GABA or Glu
- NPs can spread to distant high affinity receptors
- Different release triggers may be important for high frequency events
- If mainly from 1 brain region: may be a coordinated response
- If widespread: may not be coordinated
- Act more like 5-HT, NAd than GABA or Glu
- NPs can spread to distant high affinity receptors
- Different release triggers may be important for high frequency events
- If mainly from 1 brain region: may be a coordinated response
- If widespread: may not be coordinated
21
New cards
Neuropeptides as Drug Targets
- Peptides are poor drugs
= Susceptible to proteolysis
= Poor BBB permeability
- Some peptide systems like opioids have good small-molecule agonists and antagonists
= Endogenous opioids
= Naltrexone
= Peptidase inhibitor racecadotril treats constripation
- Drug companies have targeted peptide systems with small-molecular drugs: CRF, vasopressin, neurotensin, tachykinin
- NPs are involved in major disorders (anxiety, depression, schizophrenia) but don't translate well to clinical practice
= Susceptible to proteolysis
= Poor BBB permeability
- Some peptide systems like opioids have good small-molecule agonists and antagonists
= Endogenous opioids
= Naltrexone
= Peptidase inhibitor racecadotril treats constripation
- Drug companies have targeted peptide systems with small-molecular drugs: CRF, vasopressin, neurotensin, tachykinin
- NPs are involved in major disorders (anxiety, depression, schizophrenia) but don't translate well to clinical practice
22
New cards
Example: Corticotrophin Releasing Factor (CRF)
- 41 amino acid peptide
- Produced in hypothalamus
- Delivered to portal circulation (acts on pituitary gland to release corticotrophin)
- Released in brainstem, amygdala, BNST, cortex, etc
- Produced in hypothalamus
- Delivered to portal circulation (acts on pituitary gland to release corticotrophin)
- Released in brainstem, amygdala, BNST, cortex, etc
23
New cards
CRF Receptors
- CRF1 is widely expressed in CNS
= Ligands: CRF, Urocortin
- CRF2 is narrowly expressed in lateral and septum
= Ligands: UNC2, UNC3
= Ligands: CRF, Urocortin
- CRF2 is narrowly expressed in lateral and septum
= Ligands: UNC2, UNC3
24
New cards
CRF and depression and anxiety
- CRF is key to coordinating metabolic and behavioural responses to stress
25
New cards
Evidences for CRF and depression and anxiety
- Stress favours development of depression/anxiety
- CRF1 mutations are associated with depression/anxiety
- CRF administration in mice increases behavioural stress
- "high stress" mice have high CRF levels
- CRF1 over-expressing mice have higher behavioural stress
- CRF1 antagonists suppress behavioural changes associated with stress
- CRF1 mutations are associated with depression/anxiety
- CRF administration in mice increases behavioural stress
- "high stress" mice have high CRF levels
- CRF1 over-expressing mice have higher behavioural stress
- CRF1 antagonists suppress behavioural changes associated with stress
26
New cards
Clinical results of CRF
- Not successful overall
- Poor animal models/poor tests of stress (forced swim test)
- Animal models validated by current drugs
- Homogenous populations used in human trials (may need genetic testing to determine population in which CRF is relevant)
- No pet ligands to determine drug concentrations
- Poor animal models/poor tests of stress (forced swim test)
- Animal models validated by current drugs
- Homogenous populations used in human trials (may need genetic testing to determine population in which CRF is relevant)
- No pet ligands to determine drug concentrations