Anti-fungal and Antiviral Agents
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Why has there been an increase in fungal infections over the last 3 decades?
•Use of agents that disrupt normal host microflora - antibiotics
•Failure to develop a strong immune system or immunosuppressive drugs
Increased enormously → which predisposes to fungal infection
• Patient management that suppresses the immune response
• Chemotherapy, HIV/AIDS, transplants, steroid treatment
• Diabetes
• Antibiotic use
• Age
• Invasive surgical procedures that introduce fungi - iv lines etc
•Hospital acquired infection
What does selective toxicity mean in regard to antifungal agents?
This is where we destroy the fungus but cause no or minimal damage to the animal
To do this we need to target features of the fungus not found in the host
However, fungi are eukaryotes like us and our animals
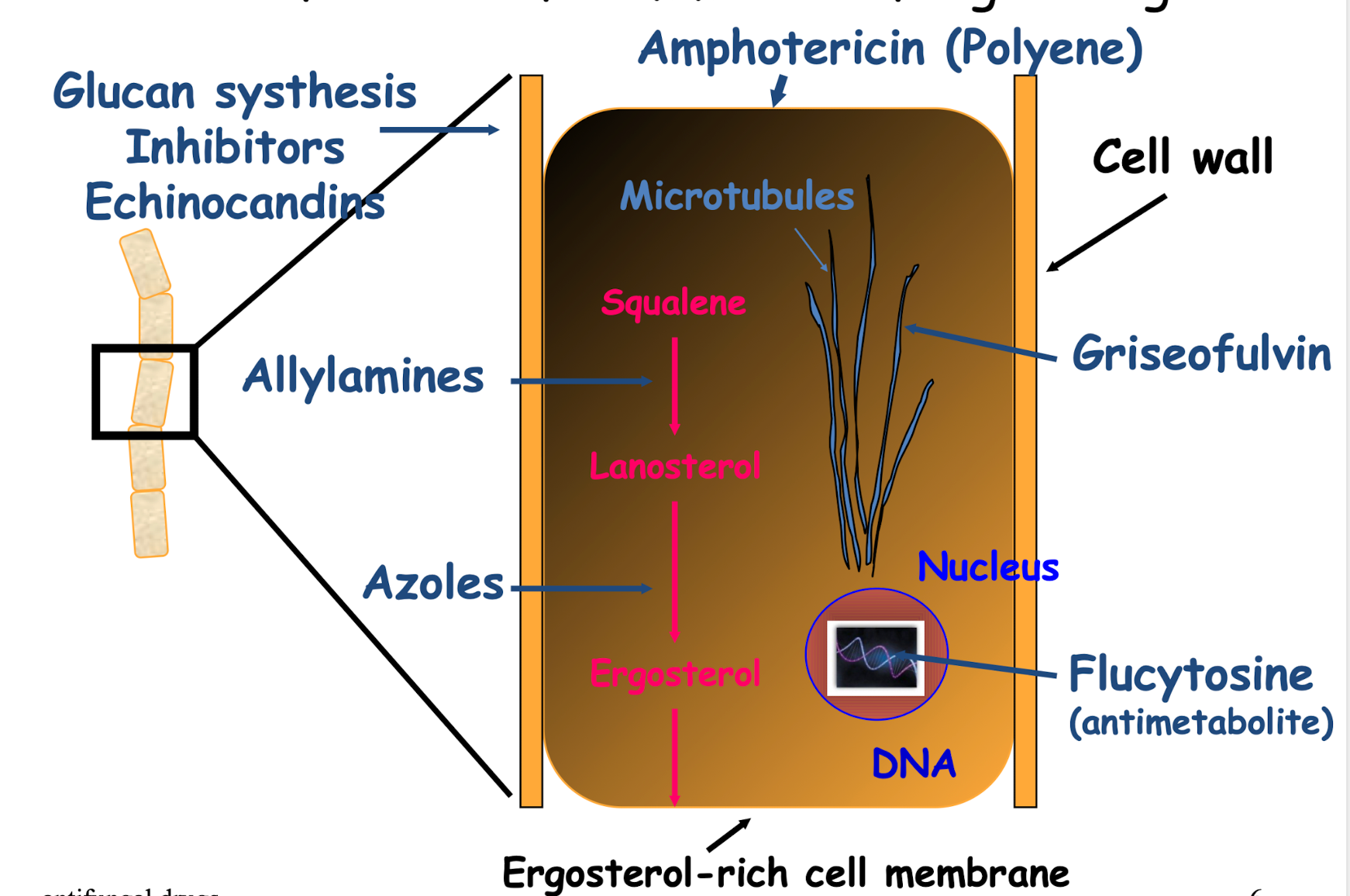
What are the main sites of action for antifungal drugs?
Fungi have a cell wall (Made of glucans and ), target for the Glucan synthesis Inhibitors Echinocandins
Main sterol is ergosterol, making them a target for the Azoles (Most common drug used to treat fungi)
Griseofulvin used to target the microtubules
How are antifungal drugs classified?
• Superficial/Systemic infection
• Topical/Systemic administration of drug
• Antifungals/ Synthetic agents
• Fungicidal/Fungistatic
Fungistatic - stops fungi from growing and the host clears it on their own
Fungicidal - used to kill fungi, used for severe infections
• Chemical Subclass
What is the chemical subclassification of antifungals?
• Allylamines
• Azoles
• Polyenes
• Glucan Synthesis (cell wall) inhibitors
• Antimetabolites
• Griseofulvin
• Other agents
What makes up the fungal cell membrane?
Ergosterol (Rather than cholesterol), is the main fungal cell membrane sterol
What three drug classes target ergosterol in the fungal cell membrane?
• Allylamines
• Azoles
• Amphotericin B (in the class called Polyenes)
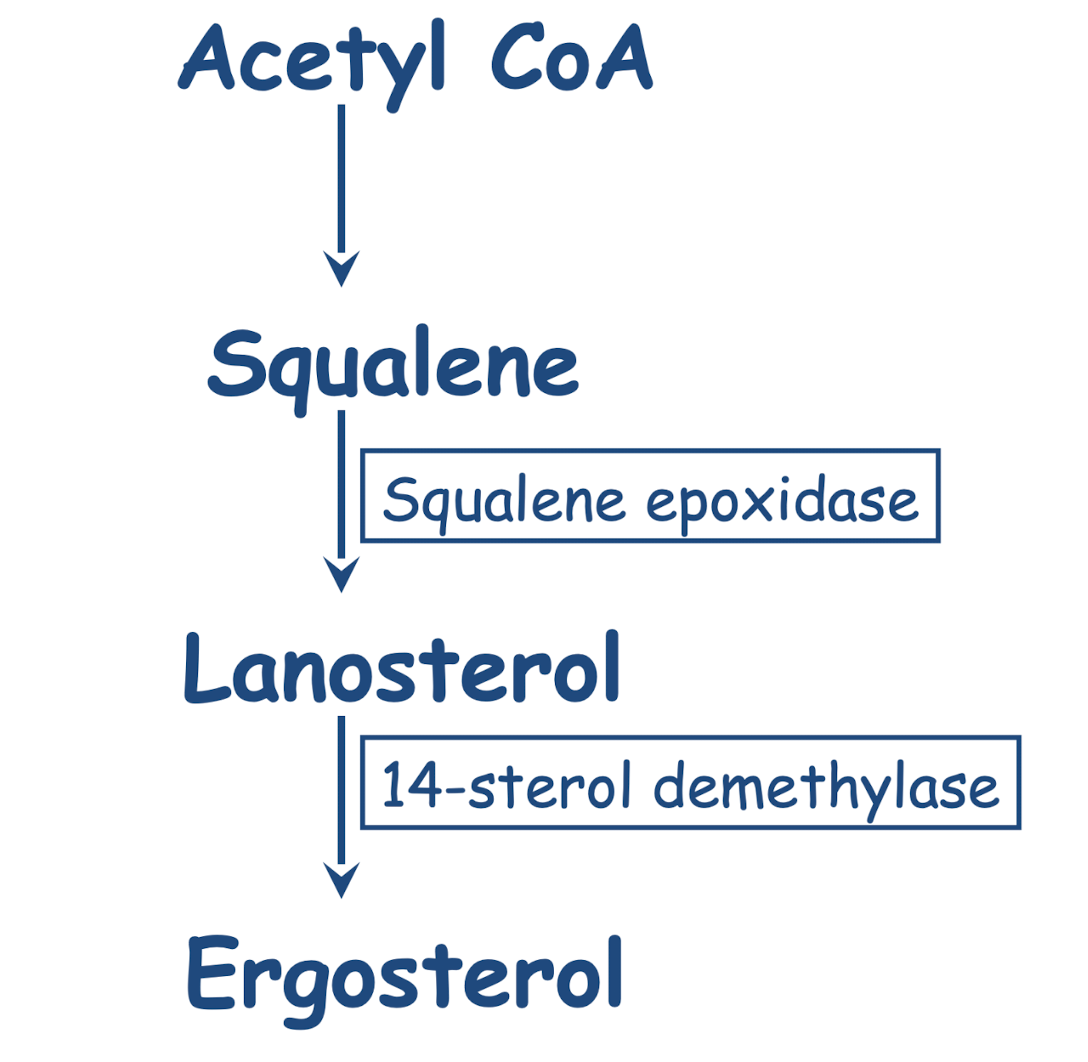
Describe the biosynthetic pathway for synthesis of ergosterol.
What are the characteristics of Allylamines?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Routes:
Pharmacokinetics:
Dermatophyte infections only, topical appl., treats hair, nails, skin
Example: Terbinafine
Mechanism of action: inhibits ergosterol biosynthesis via inhibition of squalene epoxidase (fungicidal)
Spectrum: Dermatophytes
Side effects: generally transient and mild (GIT & skin)
Routes: oral and topical
Pharmacokinetics: highly lipophilic (persists in skin)
What are the characteristics of Azoles-1?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Imidazoles - Topical use only
Examples: Clotrimazole; Enilconazole, Miconazole - lots of topical azoles - main use is topical for superticial mucous membrane and skin infection
Ketoconazole - oral therapy but toxic, may cause liver failure, but still used topically
Mechanism of action: inhibition of cytP450-dependent 14-sterol de-methylase
Side effects: GIT; anorexia; hepatotoxicity; suppression of steroid production (ketoconazole); teratogenic
What are some examples of veterinary licensed azoles?
Cats and microsporum Oral (Itraconazole)
Ear drops for dogs contains clotrimazole
For Candida, Malassezia, Microsporum, Tricophyton Rabbits, guinea pigs, rodents, birds and reptiles antifincol daira (miconazole)
For Tricophyton and Microsporum Topical (Enilconazole)
What are the characteristics of Azoles-2 - systemic?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Triazoles - for serious systemic mycoses
• fluconazole, itraconazole, voriconazole, and posaconazole
• Itraconazole - drug of choice for Histoplasmosis
• Routes: oral
• Itraconazole: lipophilic, highly plasma protein bound, hepatic metabolism and excretion in faeces
• Fluconazole: water soluble and can be given i.v, minimally plasma protein bound, minimally metabolised, 80% excreted by kidney unchanged
Describe the mechanism of resistance to azole antifungal drugs.
• Membrane changes lead to reduced drug uptake
• Mutation of the target enzyme
• Over production of the target enzyme
• Modification of the ergosterol biosynthesis pathway
• Drug efflux due to up-regulation ABC transporters, MFS transporters
• Biofilm formation
What are the characteristics of Polyenes?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Routes:
Pharmacokinetics:
• Examples: Amphotericin B, Nystatin,
• Mechanism of action: binds to ergosterol, disrupts osmotic integrity of the membrane by forming pores - ions leak from cell - cidal and oxidative damage (cells basically leak, other molecules can enter and cause damage)
• Spectrum: broad spectrum
• Side effects: nephrotoxicity (i.v.), hypokalaemia, thrombophlebitis
Fluid load the PT to reduce
• Routes: depends on preparation - Nystatin topical
• Pharmacokinetics: poorly water soluble (amphotericin B is forms a colloid in solution for injection), poor absorption from GIT
What are some examples of nystatin products?
Spectrum of nystatin is broad but topical version mainly for yeast infection in skin
For otitis externa in dogs and cats
Also contains a drug that kills Ear mites and an anti-inflammatory Drug.
What are the mechanisms of resistance to polyenes?
• Amphotericin B resistance remains rare
• Linked to reduced ergosterol in fungal cell membrane
• Forms cross membrane pores - leads to cell leakage and cell death
• Resistance linked to increased intracellular catalase reducing the oxidative killing mechanism of AmB.
• Innate resistance in some isolates of Candida lusitaniae, Aspergillus terreus, Scedosporium species, Lomentospora prolificans and Purpureocillium lilacinum.
• Fungicidal - emergent resistance rare - cells killed not bathed in fungistatic agent allowing mutation resistance - although observed in C. guilliermondii
Do fungi have a cell wall? VIDEO
Yes, fungal cells are eukaryotic, BUT have a CELL WALL
Is a selective target for antifungal drugs
What are the characteristics of Echinocandins?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Routes:
Pharmacokinetics:
IS a GLUCAN synthesis INHIBITOR
• Severe systemic mycoses only
• Examples: Caspofungin; Anidulafungin, Micafungin
• Mechanism of action: block synthesis of B (1,3) glucan
• Spectrum: Candida & Aspergillus Species
• Side effects: minimal
• Routes: i.v.
• Pharmacokinetics: water soluble, highly plasma protein bound, eliminated in urine and faeces as metabolites
What are the mechanisms of resistance to echinocandins? VIDEO
• Inhibit glucan synthase which synthesizes beta glucan, a structural component of fungal cell walls
• Structural integrity lost - cidal in yeast, static in moulds
• Many moulds resistant (not Aspergillus) most Candida species susceptible
• Candida parapsilosis innately less susceptible - higher breakpoints
• Various FKS1 (glucan synthase subunit) mutations identified also FKS2 and 3 genes all of which encode the target enzyme and up-regulation of chitin synthesis - rescue mechanism
What are the characteristics of Antimetabolites?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Routes:
Pharmacokinetics:
• Examples: Flucytosine (5-fluorocytosine - converted to 5-fluorouracil by cytosine deaminase) - Combination therapy for severe yeast infections only
Should not be used on its own as resistance will occur
• Mechanism of action: Incorporation of 5-FU into RNA disrupts protein synthesis (fungicidal) and further conversion of 5-FU to fluorodeoxyuridine monophosphate inhibits thymidylate synthase which interferes with DNA synthesis
• Spectrum: Narrow; Cryptococcus; Candida species
• Side effects: generally well tolerated
• Routes: oral
• Pharmacokinetics: excreted unchanged by kidney
• Often combined with Amphotericin B (synergism)
Describe the mechanisms of resistance to flucytosine (5-FC).
• Sensitive - cascade of active enzymes, cytosine permease to take up the drug, cytosine deaminase and phosphorylase to metabolize it to its toxic form - disrupts RNA and DNA synthesis
• 5-FC converted to 5-FU - Fluorouracil - miscoded RNA or DNA
• Loss or reduction in activity in any of the enzymes (but most commonly the phosphorylase) leads to primary emergent resistance
• Common during treatment - rarely used as monotherapy, always combined with AmB
What are the characteristics of Griseofulvin?
Example:
Mechanism of action:
Spectrum:
Side Effects:
Routes:
Pharmacokinetics:
Dermatophyte infections only
Mechanism of action: selectively deposited in newly formed keratin, inhibits mitosis, disorganises the spindle microtubules (fungistatic)
Spectrum: Narrow spectrum (dermatophytes)
Side effects: idiosyncratic reaction in cats, teratogenic
Idiosyncratic - unpredictable, has not occurred in other animals = unexpected side effects in cats
Routes: Oral (with high fat diet)
Pharmacokinetics: Poorly water soluble, hepatic metabolism and faecal elimination
Note: No longer licenced for food producing animals in UK. Only horses.
What are some other agents that might be used as antifungals?
Iodides
Propionic, Salicylic and Undecanoic Acids
Phenolic Antiseptics
• Iodides - may enhance the immune response of the host
• Propionic, salicylic and undecanoic acids
• Whitfield's ointment: benzoic acid and salicylic acid in an emulsifying base) have been used traditionally for treating dermatophyte infections of the skin. Though old-fashioned and a little messy, they are cheap and effective.
• Phenolic antiseptics
- e.g. thymol
- Hexachlophene
What are some combination preparations of antifungals?
Eg for ear infection
Contains:
• Marbofloxacin
•Clotrimazole
• Dexamethasone
Antiviral drugs
VIDEO
What is the purpose of antiviral therapy?
Not many anti-virals for animal disease but becoming more available for human medicine - (therapy for HIV, Hepatitis B and C virus)
Drugs for Feline Herpesvirus 1 - ocular keratitis - acyclovir ophthalmic ointment (Idoxuridine/trifluoridine also)
FIV - feline immunodeficiency virus - zidovudine - nucleoside analogue that blocks viral reverse transcriptase
Canine herpesvirus 1 - ocular lesions - Idoxuridine/trifluoridine
Canine parvovirus 2 - interferon-w
Equine herepesvirus 1 - valacyclovir?
Influenza - experimentally effective in animals but used in man
Targets - foot and mouth disease, bluetongue, classical swine fever, bovine viral diarrhoea virus
Do not exist in clinical practice
What are antiviral drugs targeting?
Antibodies
Ion channel blockers
Reverse Transcriptase inhibitor
DNA polymerase
Neuraminidase inhibitor
What is Aciclovir? How does it function as an antiviral agent?
Aciclovir:
- inhibits viral DNA polymerase
- High specificity for herpes simplex
- oral, iv, topical - wide distribution
- minimal side effects
- Uses FHV, viral eye infection
What is Amantadine & Rimantadine? How does it function as an antiviral agent?
Amantadine and Rimantadine:
- blocks viral M2 ion channel
- Influenza A
- oral - well absorbed
- minimal side effects
What is Zidovudine (AZT)?
• nucleoside reverse transcriptase inhibitor
retroviruses
oral, iv.
short term: minor reversible
side effects
;ong term: anaemia. GIT
disturbances
FLV and FIV
What is Zanamivir (Relenza) and Oseltamivir (Tamiflu)?
• Treatment and prophylaxis of Influenza A and B
• Neuraminidase inhibitor; stops new viruses emerging
Neuraminidase is needed to cleave off new virions which leave to locate a new host
• Recently used against H1N1 and H5N1
• Also active against canine parvovirus, feline panleukopenia, kennel cough, and 'canine flu'.