pH scales
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
pH definition
pH is a measure the concentration of hydrogen ions.
pH measures the acidity or basicity (alkalinity) of a substance.
pH scale
pH scale: 0-14
pH < 7 : acid
pH of 7: neutral
pH > 7 : base (alkaline)
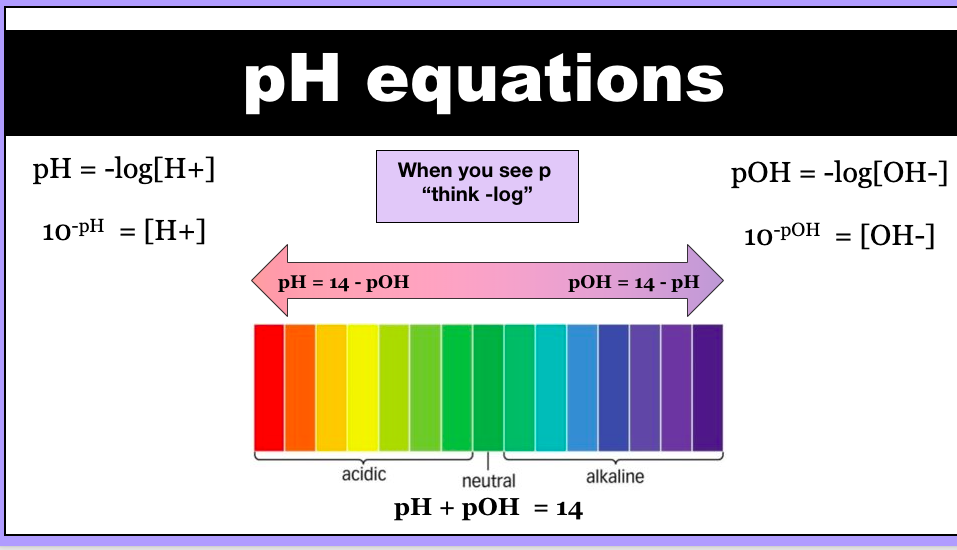
pH equation
pH is the “negative logarithm of” the hydrogen ion concentration
pH = -log[H+]
This means if the pH changes by 1, there’s 10 times difference in the concentration
If the pH changes by 2, there’s a 100 times difference in the concentration
If the pH changes by 3, there’s a 1000 times difference in the concentration
log rules
Equation to calculate pH
pH = -log[H+]
Equation to calculate concentration of H+ ions
10-pH = [H+]
concentration will be in molL/if it is the square brackets, which means it is also in molL.
pOH
a measure of hydroxide ion (OH-) concentration.
measure of the alkalinity of a solution
pOH less than 7 are alkaline, pOH greater than 7 are acidic and pOH equal to 7 are neutral. O PPOSITE to pH! There are several ways to define acids and bases, but pH and pOH refer to hydrogen ion concentration and hydroxide ion concentration, respectively.
what does the “p” stand for?
"negative logarithm of" and is used to make it easier to work with extremely large or small values. pH and pOH are only meaningful when applied to aqueous (water-based) solutions.
relating pOH and pH
Calculate the pH of a 0.2 mol L–1 solution of sodium hydroxide.