Physics- Topic 23 ( Nuclear Physics)
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Energy and Mass Equivalence
E = mc2 where E= energy, m= mass and c = speed of light
Representing simple nuclear reactions
AZX where A: nucleon number/mass number, Z= atomic number/ proton number and X= chemical symbol of the element
Mass defect
The difference between an atom’s mass and the sum of the masses of its protons and neutrons
∆m = Zmp + (A-Z) mn - mtotal
where:
Z = proton number
A = nucleon number
Mp= mass of a proton
Mn = mass of a neutron
Mtotal = measured mass of a nucleus
Binding energy
The energy required to break a nucleus into it’s constituent protons and neutrons
Energy released in nuclear reaction
E = c2m
The formation of a nucleus from a system of isolated protons and neutrons is therefore an exothermic reaction
Binding energy per nucleon
The binding energy of a nucleus divided by the number of nucleons in their nucleus
A higher binding energy per nucleon indicates a higher stability
Graph of number of nucleons vs the binding energy per nucleon
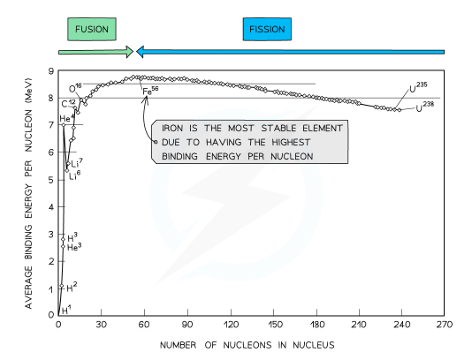
At high values of nucleon number
The general binding energy per nucleon is high & gradually decreases with nucleon number
This means the heaviest elements are volatile and undergo fission
At low values of nucleon number
The nuclei tend to have a lower binding energy per nucleon, hence they are generally less stable
This means thatthe lightest elements have weaker electrostatic forces and most likely undergo fusion
NOTE: Oxygen, Carbon, and Helium do not fit the trend
Nuclear fusion
Fusion is the fusing together of two small nuclei to produce a large nuclei nucleus
For two nuclei to fuse, both nuclei must have high kinetic energy
This is because protons repel each other
Eg: Deutrium + tritium → Helium + energy
Nuclear fission
Fission is the splitting of large atomic nuclei into smaller nuclei
Fission must be induced by firing neutrons at a nucleus
When the nucleus is struck by a neutron, it splits into 2 or more daughter nuclei and ejects neutrons which can react with another nuclei causing a cascade effect
This reaction needs to be controlled otherwise it can cause a nuclear bomb effect
Significance of binding energy per nucleon
At Lower values of A
Attractive nuclear forces between nucleons outweigh repulsive electrostatic forces between protondprotons
In the right conditions, nuclei undergo fusion
In fusion, the mass of nucleus that is created is slightly less than the total mass of the original nuclei
The mass defect is equal to the binding energy released since the nucleus formed is more stable
At higher values of A
Repulsive electrostatic forces begin to dominate, and these forces tend to break apart the nucleus rather than hold together
In the right conditions, nuclei will undergo fission
In fission, an unstable nucleus is converted to a more stable nuclei with a smaller total mass
Mass defect = binding energy
Radioactive decay
Is spontaneous disintegration of a nucleus to form a more stable nucleus, resulting in the emission of an alpha, beta or gamma particle
Evidence for the random nature of radioactive decay
This can be observed with the count rate of a Geiger-Muller(GM) tube
When a GM tube is placed near a radioactive source, the counts are found to be irregular and can’t be perdicted
Each count represents a decay of an unstable nucleus
These fluctuations in count rate on the GM tube provide evidence for the randomness of radioactive decay
Characteristics of radioactive decay
Spontaneous: A process which can not be influenced by environmental factors
Random: A process in which the exact time of decay of a nucleus can not be predicted
Decay constant
Is the probability that an individual nucleus will decay per unit time
Average number of nuclei which are expected to decay per unit time
Activity
Is the rate at which radioactive decay occurs
A = ΔN/ Δt = -λN
Where:
A = activity
λ = decay constant
ΔN = number of undecayed nuclei
Δt = time interval
N = number of nuclei remaining in a sample
The minus sign shows that the number of undecayed nuclei decreases over time
Half-life
Is the time taken for the initial number of nuclei to reduce by half
This means when a time equal to half-life has passed, the activity of the sample will also have decreased by half.
A ∝ N
λ = 0.693 / t1/2
Exponential nature of radioactive decay
In radioactive decay, the number of nuclei falls very rapidly without ever reaching zero
Such a model is known as exponential decay
The steeper the graph is, the larger the decay constant
General Equation
X = X0e-λt where X can be activity, number of undecayed nuclei or received count rate