Biology Lecture - Final Exam - Study Guide
1/484
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
485 Terms
What to think of when drawing dot diagrams
think of only valence shell and trying to fill valence shell
covalent bonds
strongest bond. bond where the elements are sharing valence electrons
electronegativity
propensity of an atom to pull on electrons. electrons are in a cloud around the nucleus and protons pull on this cloud
factors that affect electronegativity
1. how many protons there are
2. how close the electrons are
elements with the highest electronegativity
oxygen and fluorine
carbon and hydrogen are:
have similar enough electronegativities that where there is not much of a difference between them
relative electronegativities tell us:
if a covalent bond is polar or nonpolar
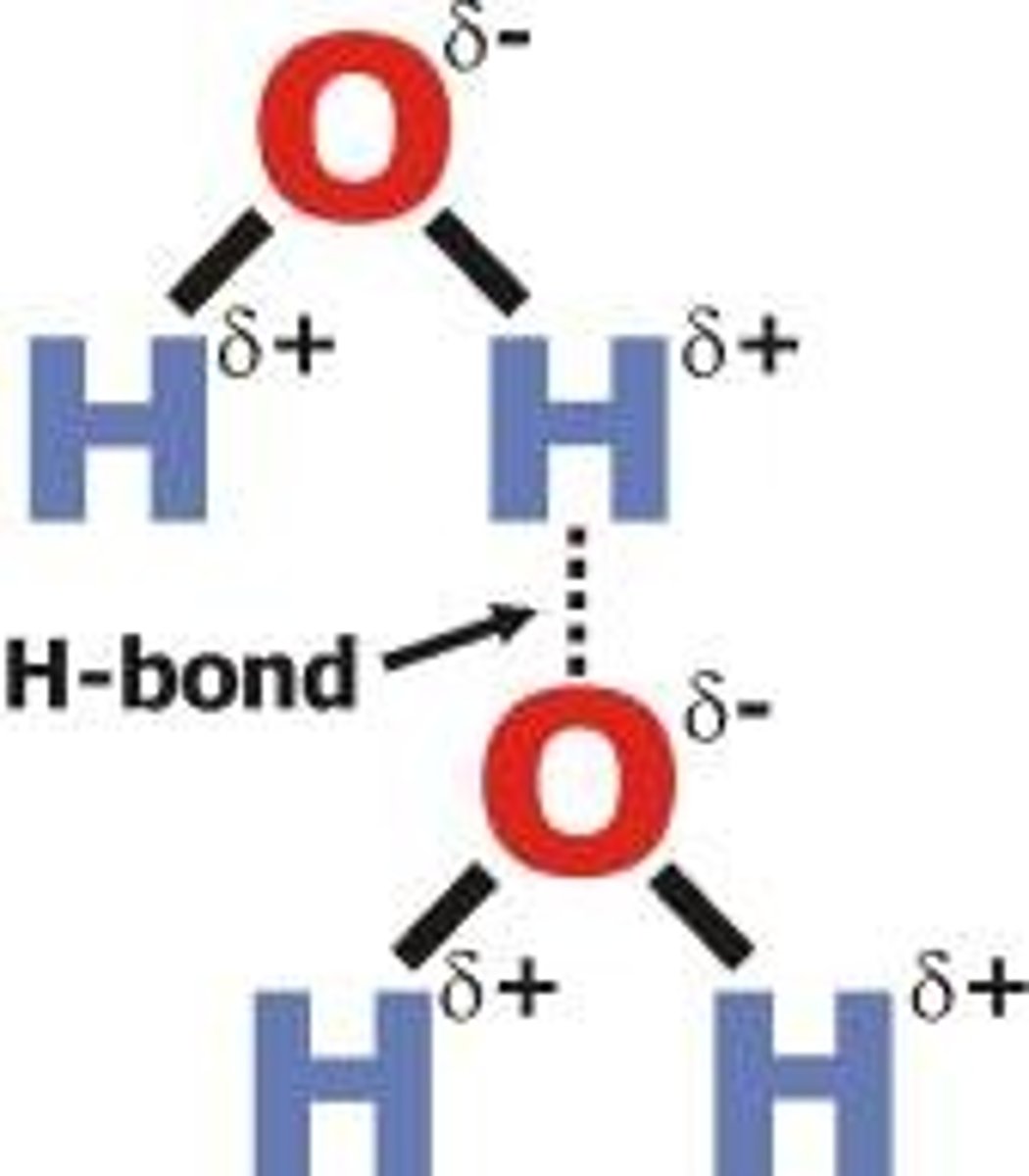
oxygen in water
oxygen has a higher electronegativity and is better at pulling electrons towards its nucleus. causes it to have slightly more negative charge (partial negative)
hydrogen in water
hydrogen has a lower electronegativity and is worse at pulling electrons towards its nucleus. causes it to have slightly more positive charge (partial positive)
noticeable difference in electronegativity
polar bond
unnoticeable difference in electronegativity
nonpolar bond
hydrogen bond
an attraction between a partial negative on one molecule and a partial positive on another molecule or in different regions of the same molecule
H+
1 proton with 0 electrons
acid
a substance that increases the hydrogen ion concentration of a solution. when an acid is in a solution it can give off an H+
Strong acids
HCl, H2SO4, HNO3
HCl
H+ + Cl-
H2SO4
H+ + HSO4-
HNO3
H+ + NO3-
factors that affect the strength of an acid
1. Strength of the HA bond
Strength of the HA bond
the more polar the H-A bond, the more likely it is for H+ to come off. if the electron is spending more time with another element, then the hydrogen is more likely to come off of the molecule
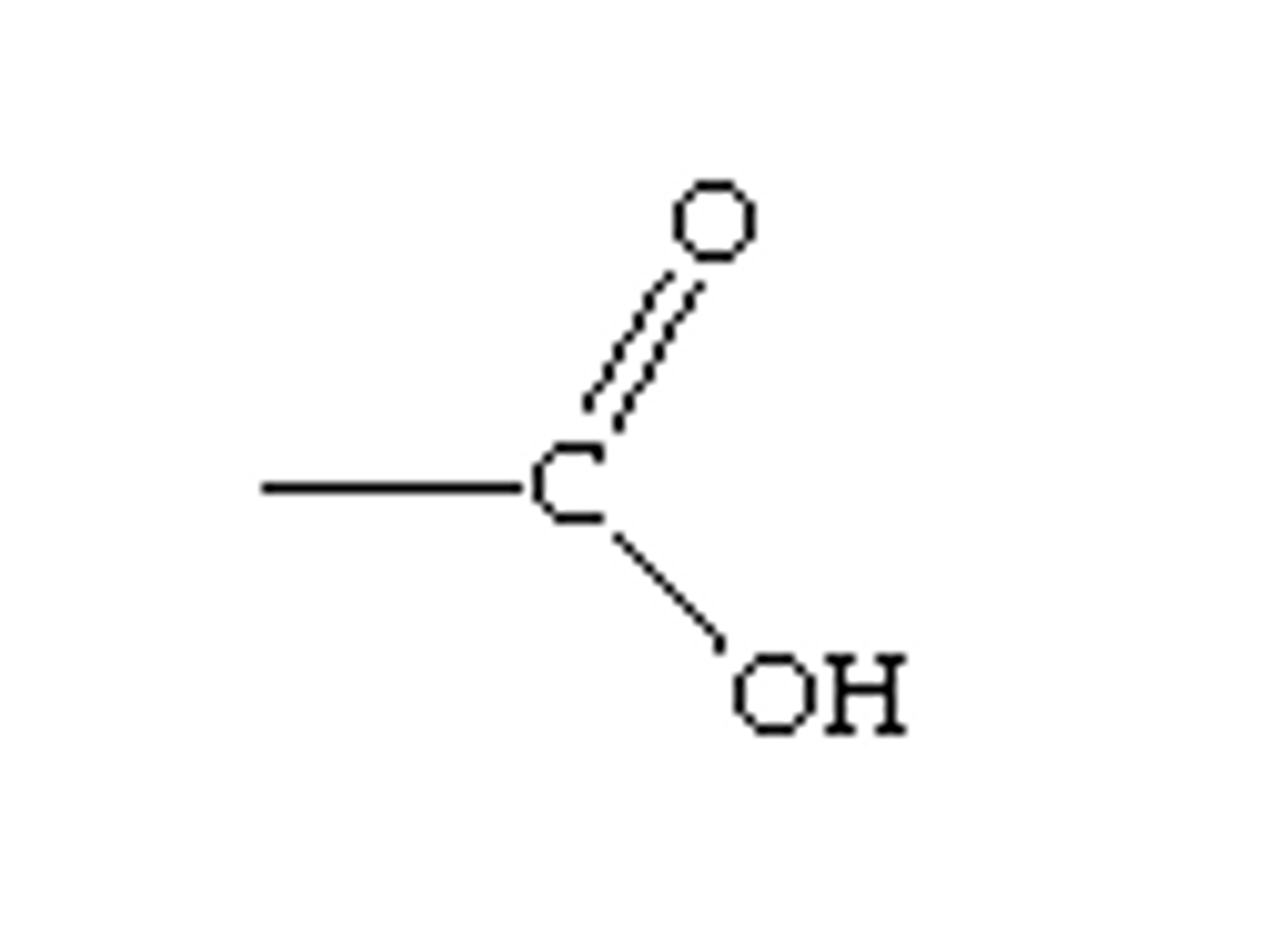
COOH
high electronegativity of O double-bonded to C causes more electrons to move towards O. (double bonds means it is dealing with twice the amount of electrons) O attached to H is going to pull especially hard on electrons of H because the other O is pulling so hard on C's electrons already.. This makes it a weak acid (H sometimes comes off)
COOH
carboxylic acid (commonly found in organic acids)
anything that has carboxyl will:
function as a weak acid
bases
A substance that reduces the concentration of hydrogen ions in a solution
common bases
NaOH, NaHCO3, NH3
NaOH
Na+ + OH-
NaHCO3
NaHCO3 + H+ --> Na+ + H2O + CO2
NH3
NH3 + H+ -> <- NH4+
Weak acid in water
CH3COOH + H2O <- -> CH3COO- + H+
Weak base in water
NH3 + H2O -> <- NH4+ + OH-
pH scale
-log[H+]
Why does the concentration of H+ matter
it affects the structure and form that an amino acid is in. an amino acid could be charged differently in solutions with a high or low pH
functional groups
groups of atoms commonly found together in molecules of living things. when present they have an effect on the molecule
carbon is important because
carbon can make 4 bonds and makes a great foundation for molecules
carbon line and ring drawing
end or bend is a carbon. carbon forms 4 bonds, so any bond not shown is a hydrogen
how to determine how a functional group will impact molecule function
Is it polar?
is it an acid or negatively charged?
is it a base or positively charged?
hydroxyl group
present in alcohols. the oxygen will have a partial negative and the hydrogen will have a partial positive
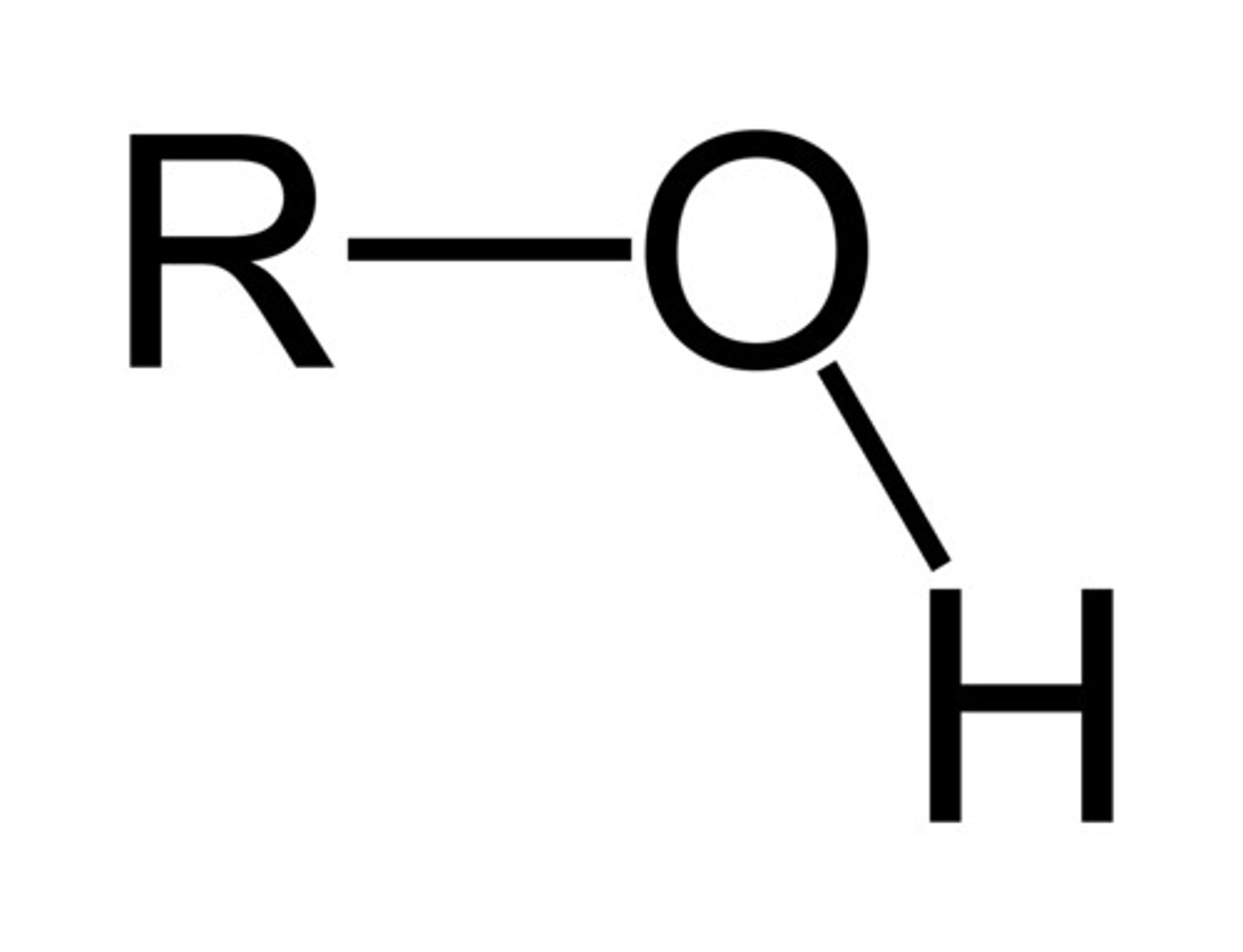
hydroxyl group questions
Is it polar? - yes
is it an acid or negatively charged? - no (pull is not strong enough because of what the OH is attached to)
is it a base or positively charged? - no
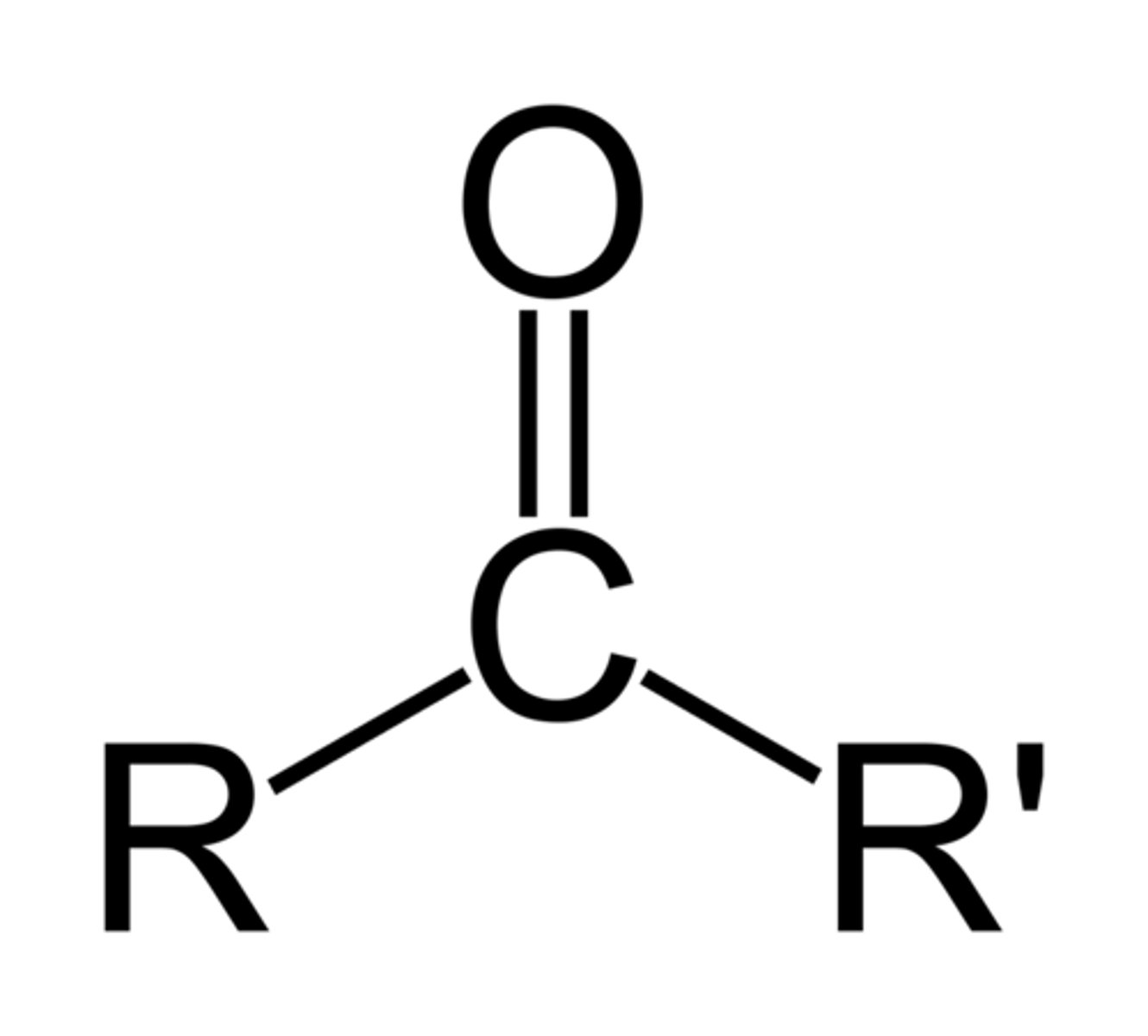
carbonyl group
double bond between carbon and oxygen, so its a very polar bond
carbonyl group questions
Is it polar? - yes
is it an acid or negatively charged? - no
is it a base or positively charged? - no
forms of carbonyl
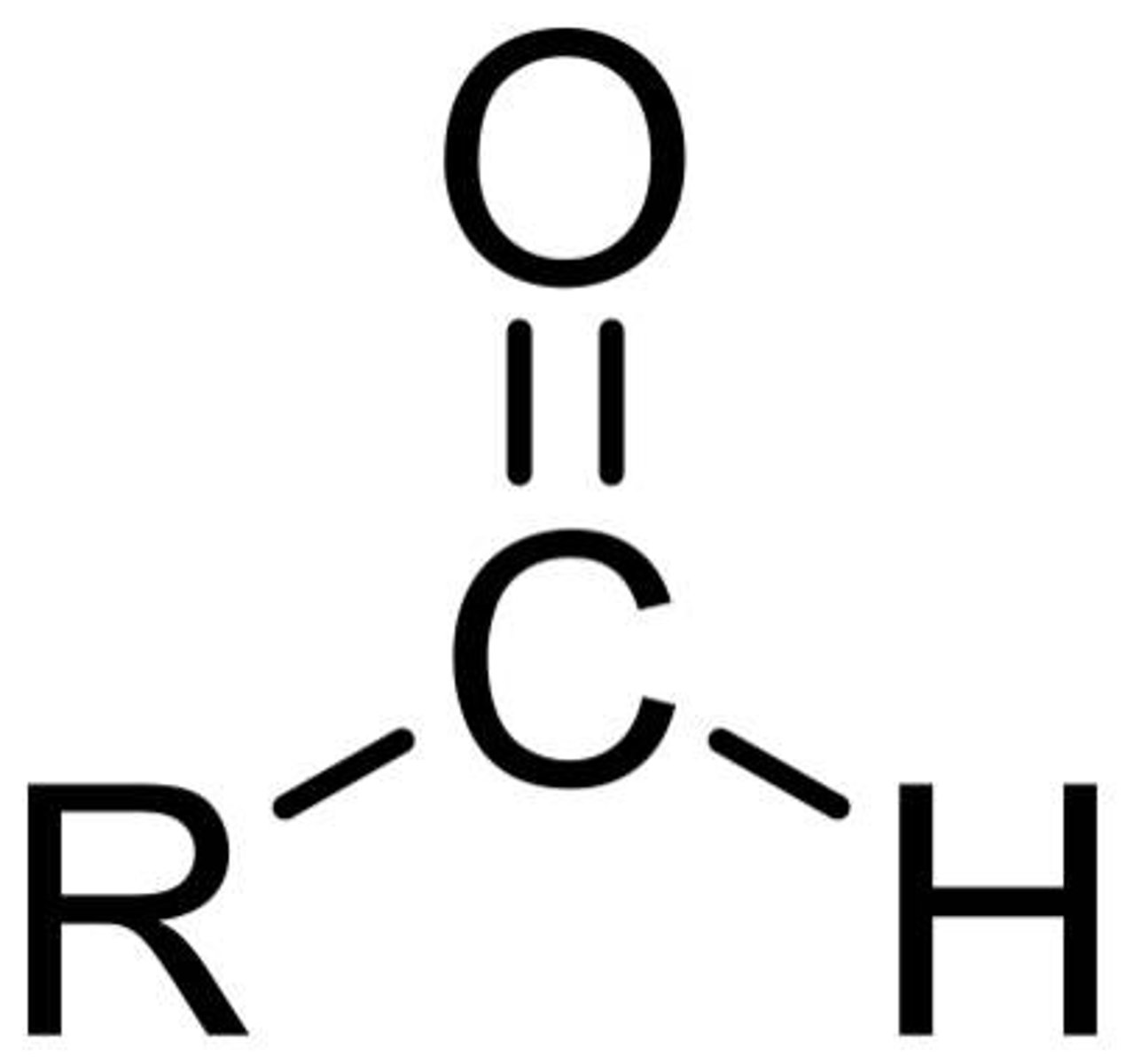
ketone and aldehyde
what is this?
carbonyl ketone
what is this?
carbonyl aldehyde
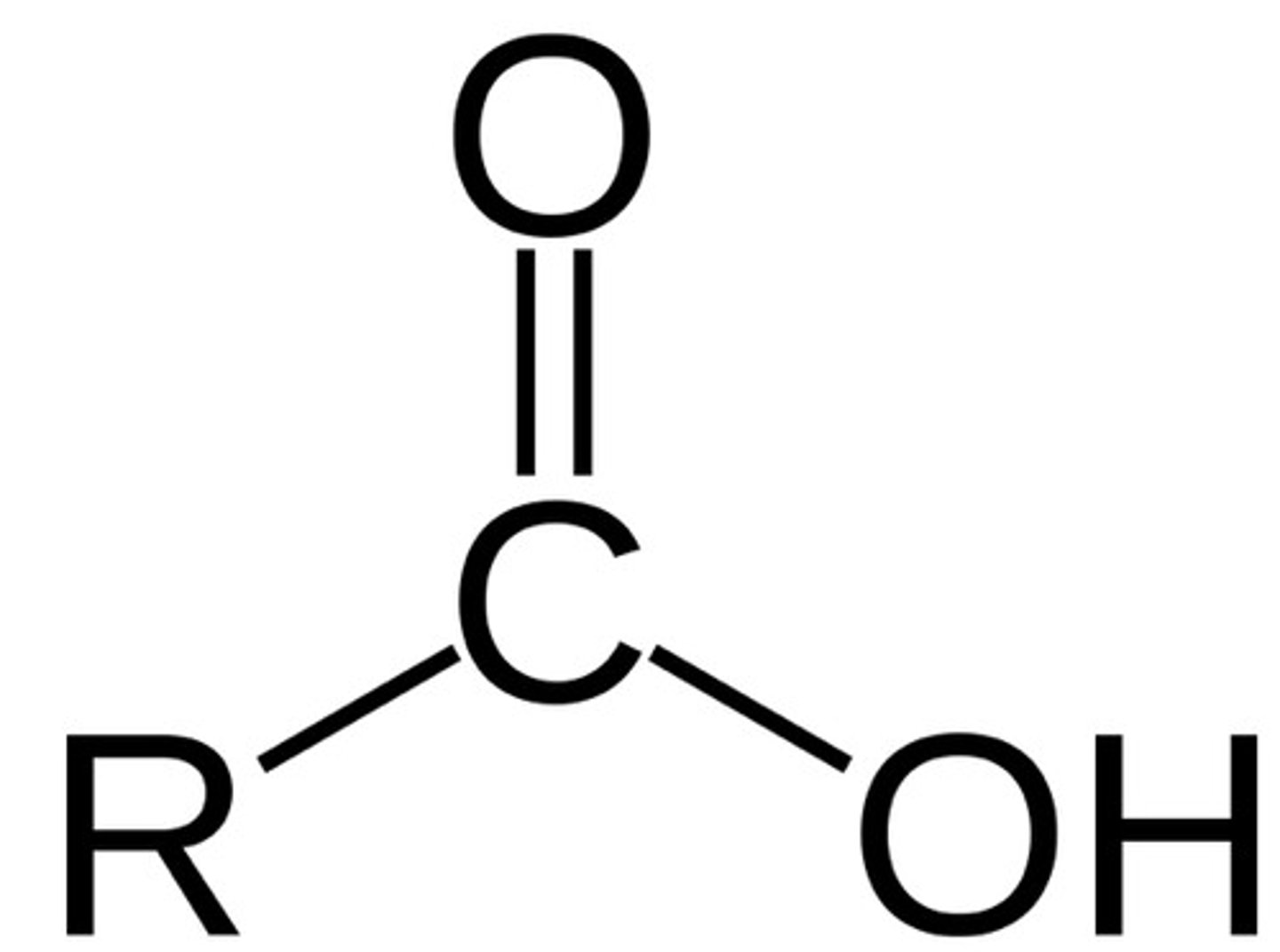
carboxyl (carbonyl + hydroxyl but is an independent molecule)
Is it polar? - yes
is it an acid or negatively charged? - yes
is it a base or positively charged? - no
what is this
amino group
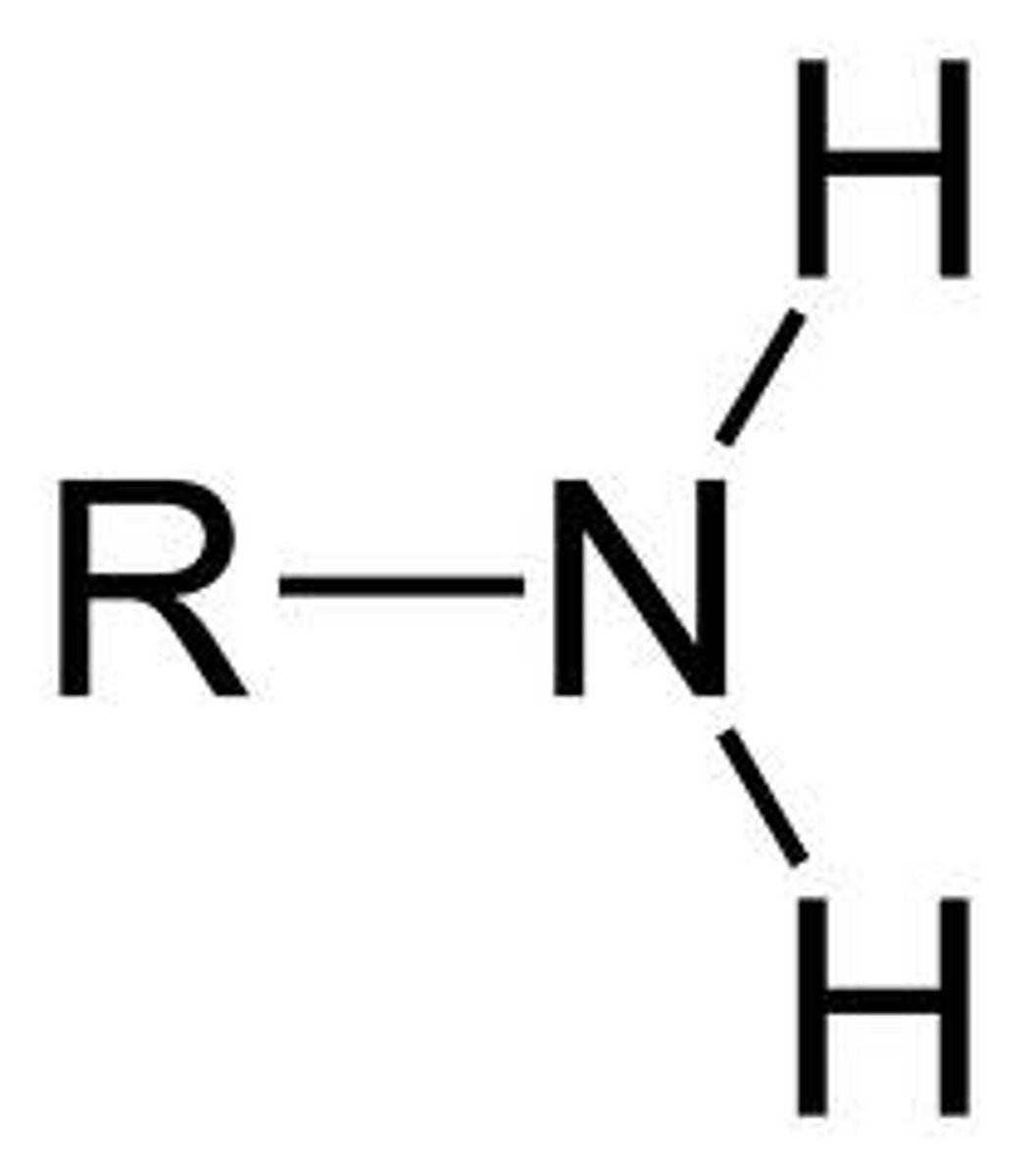
amino group questions
Is it polar? - yes
is it an acid or negatively charged? - no
is it a base or positively charged? - yes
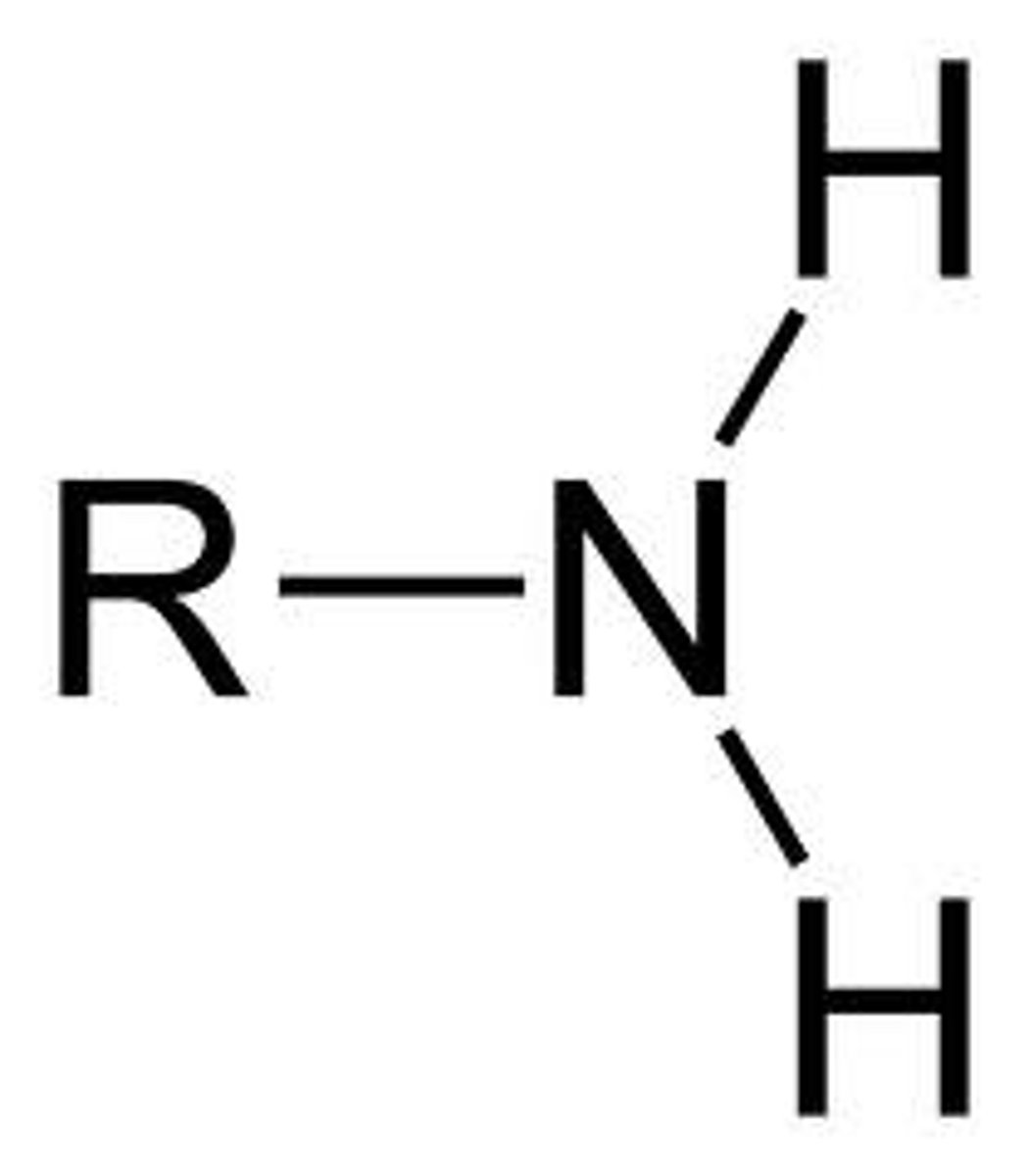
what is this
methyl group
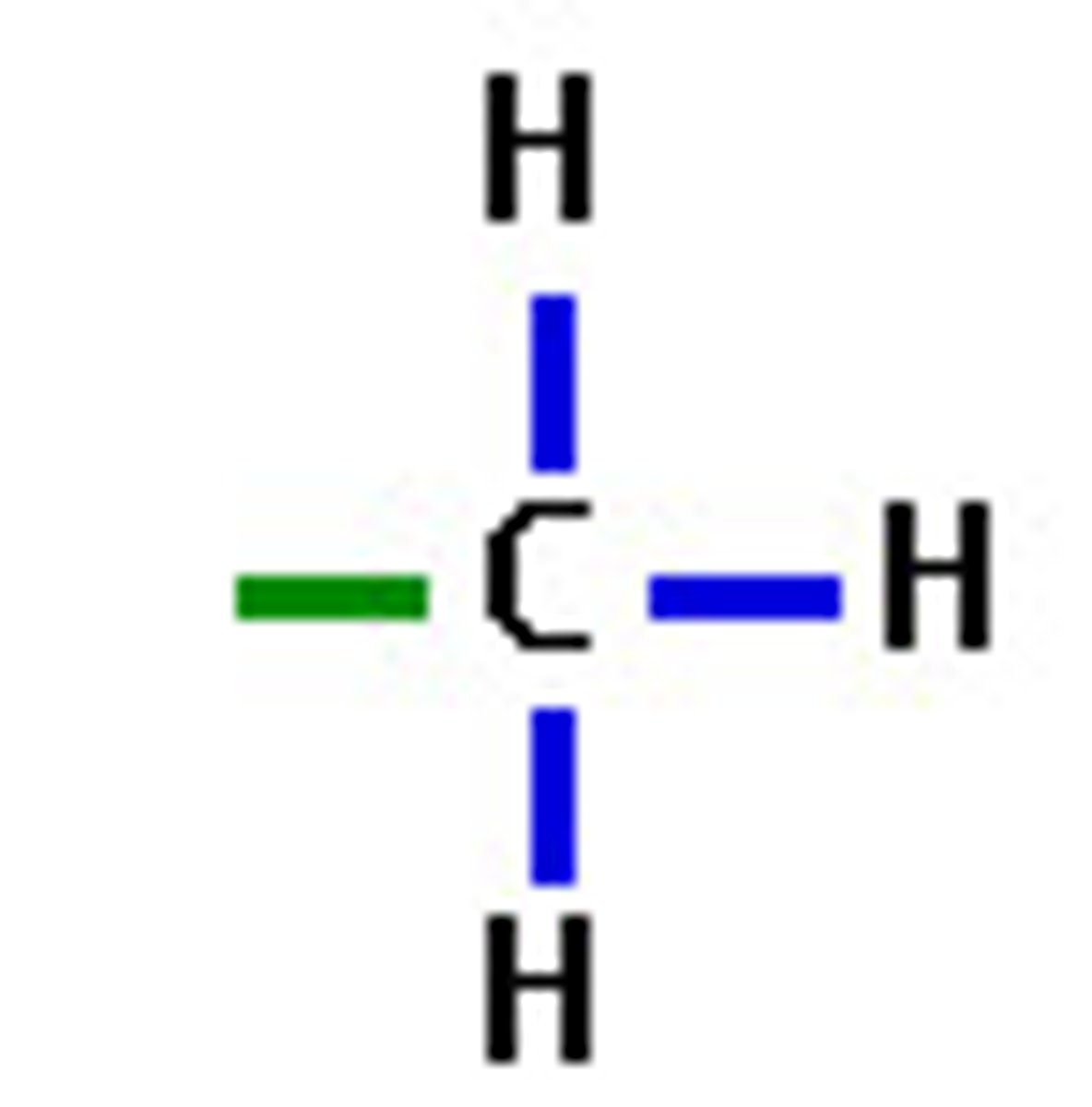
methyl group
Is it polar? - no
is it an acid or negatively charged? - no
is it a base or positively charged? - no
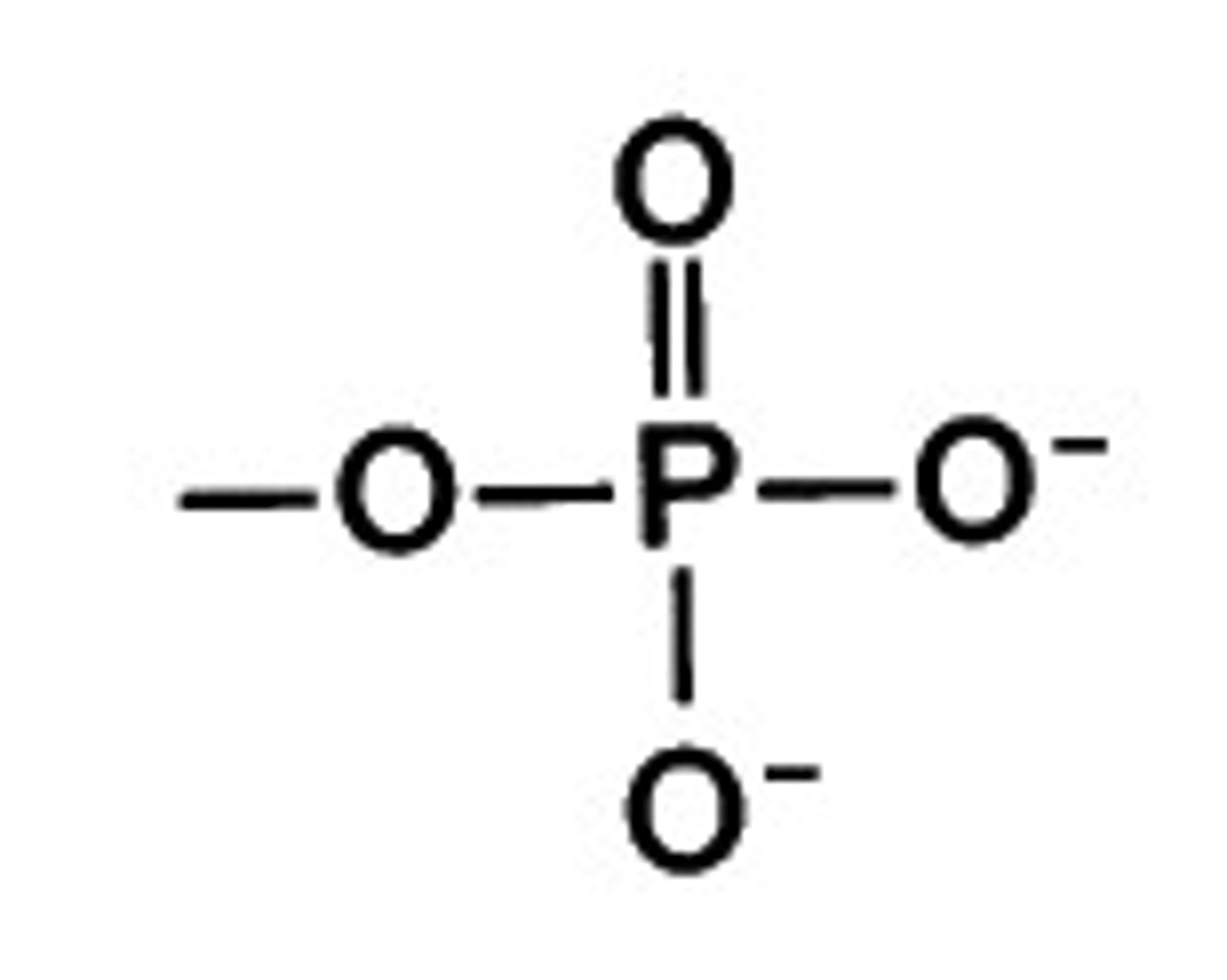
phosphate group questions
Is it polar? - yes
is it an acid or negatively charged? - yes
is it a base or positively charged? - no
what is this
phosphate group
alpha carbon
carbon that is bonded to both the amino and carboxyl groups in an amino acid
what reaction creates a bond between two amino acids?
dehydration synthesis
what is the resulting polypeptide of the amino acids bonding called?
amino acid residues (entirety of both molecules is not there anymore)
what is the bond between two amino acids?
peptide bond
what is the first structure of polypeptides?
primary structure
define primary structure
a sequence of polypeptides that form the base of that protein. held together by covalent peptide bonds.
define secondary structure
forms via hydrogen bonds within the polypeptide backbone, forming folded and helical shapes
hydrophobic interactions
nonpolar things interacting with other nonpolar things
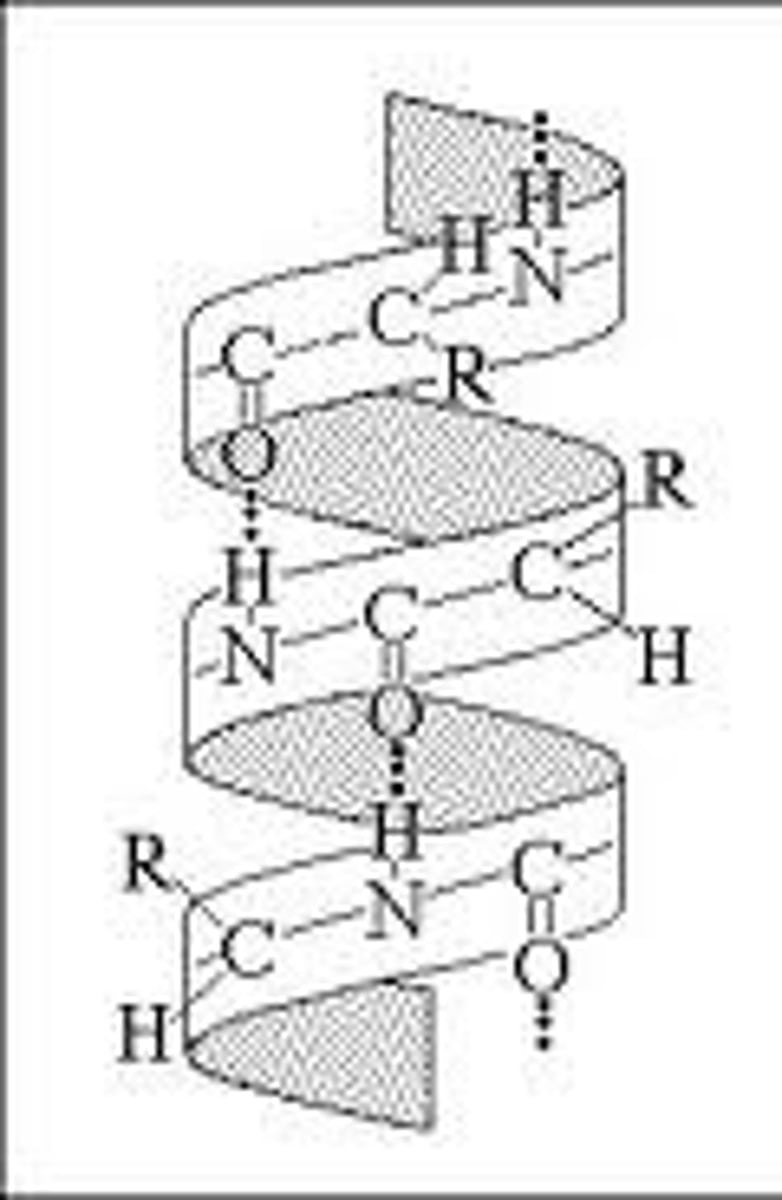
types of secondary structures
alpha helix and beta pleated sheet
what is this
alpha helix
what is this
beta-pleated sheets
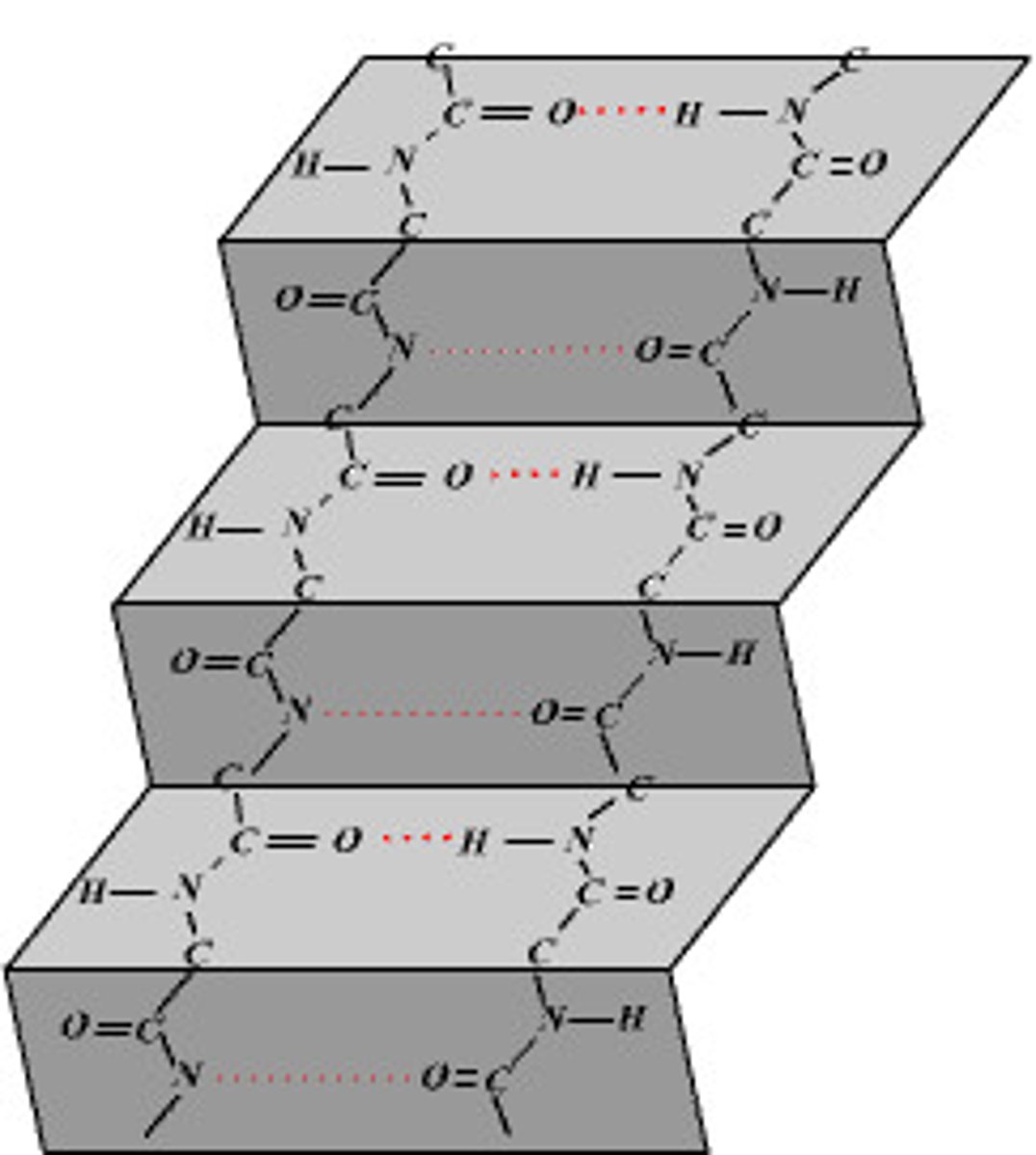
define a tertiary structure
tertiary structure refers to the three-dimensional folding of a protein due to interactions between R-groups of the same polypeptides
quaternary structure
not all proteins have this. same bonds as tertiary structures. formed via R-group interactions of different polypeptide chains (multiple chains)
hydrogen bonds
weak attraction between a hydrogen atom and another atom (partial charges attracted to one another)
ionic bond
A chemical bond resulting from the attraction between oppositely charged ions.
disulfide bridges
A strong covalent bond formed when the sulfur of one cysteine monomer bonds to the sulfur of another cysteine monomer.
types of bonds/interactions that make a quaternary and tertiary structure
disulfide bridges, hydrogen bonds, ionic bonds, hydrophobic interactions
protein denaturation
process in which a protein loses its 3D structure
things that denature proteins
heat above 50 C, alcohols, salts of heavy metals, alkaloid reagents
heat above 50 C
heat increases means increased kinetic energy, disturbing hydrogen bonds and hydrophobic interactions
alcohols
these form their own hydrogen bond due to OH. this disrupts the intramolecular bonds
salts of heavy metals
forms strong bonds with acidic R-groups
alkaloid reagents
combine with positively charged R-groups. also disrupts ionic bonds
why does water travel thru aquaporins so well reason 1:
Residual carboxyl groups from the backbone form H-bonds with incoming water make a conduction pathway
why does water travel thru aquaporins so well reason 2:
Two asparagine R-groups cause water to turn in the middle of the channel
why does water travel thru aquaporins so well reason 3:
R-groups with rings create a selectivity filter blocking molecules larger than water
How does the structure of insulin facilitate its function? reason 1:
Disulfide bridges between cystines make a stable protein that can travel through the bloodstream
How does the structure of insulin facilitate its function? reason 2:
Shape of insulin facilitates binding to insulin receptor
How does the structure of insulin facilitate its function? reason 3:
Histidines bind to zinc allowing insulin to be stored as a hexamer
c-peptide in insulin
a chain that is cut out and sometimes used to determine metabolic disorders
what is the relationship between a protein and its receptor?
they are complementary in structure and charge, allowing for a perfect fit
insulin
a hormone that regulates glucose levels within bloodstream by converting it into glycogen and storing it in the liver
insulin triggers...
movement of glucose into cell via a cascade and glucose transporter
___ allows insulin to bind to ____
histidine; zinc
aquaporin
a channel protein that is embedded into a membrane and allows water to move into cell (passive transport)
what does water do as it moves through aquaporins?
turns 180 degrees for efficiency and it allows for water to move in a single line
if you have nitrogen, the R-group always a base unless:
it has a C double-bonded to an O
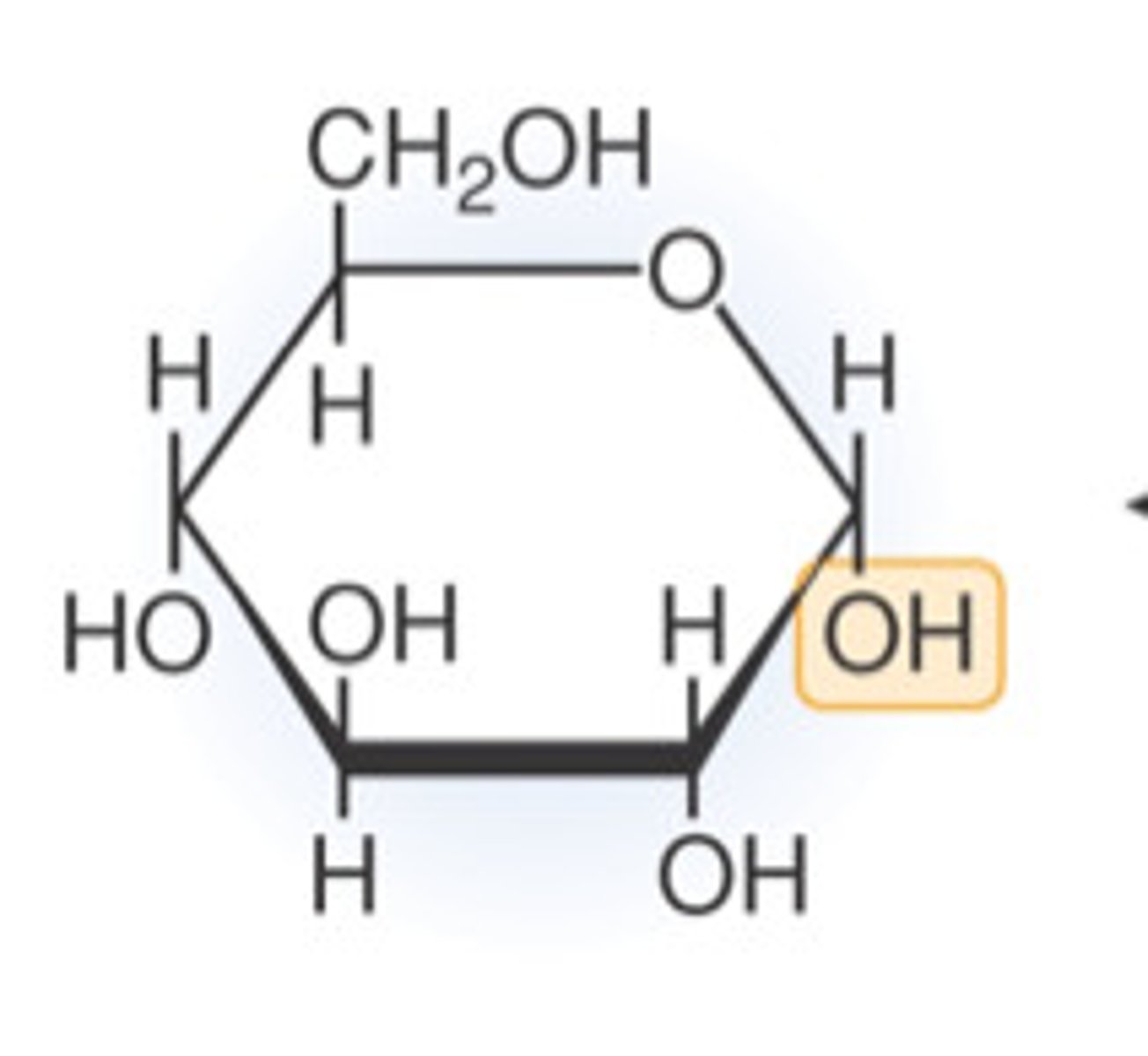
glucose can either be drawn as..
linear or ring
normally simple carbs follow this formula:
(CH2O)*n
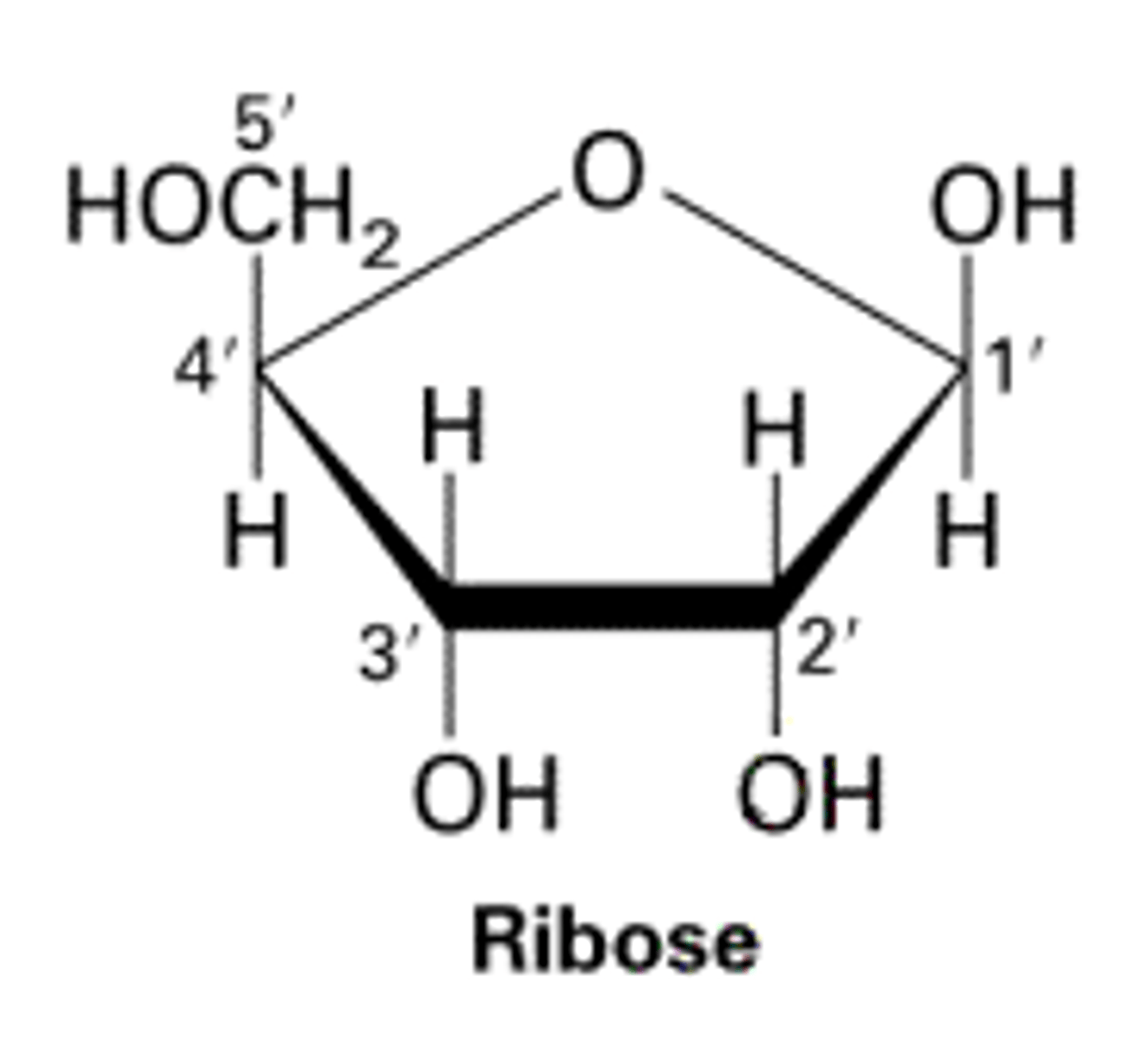
ribose
A five-carbon sugar present in RNA
functions of carbs in living things
short-term energy storage, used in plant structure, involved in information molecules, cell to cell recognition
what is an example of a carb used for short-term energy storage
glucose
what is an example of a carb used for structure
cellulose
what is an example of a carb used for information molecules
ribose or deoxyribose in DNA or RNA
what is an example of a carb used for cell to cell recognition
carbs on surface of membrane, key in recognition
things to keep in mind when drawing dehydration synthesis reaction between amino acids
1. it must be a bond between the amino terminal and carboxyl terminal
2. 1 H from NH2 and the OH from COOH is taken out to form H2O
3. The NH2 and COOH that are involved must be attached to the alpha carbons of its respective amino acid
hydrophobic R-groups are composed of which atoms
carbon and hydrogen
acidic R-groups contain ____
carboxyls
what bonds are acidic amino acids likely to form in tertiary structure?
ionic and hydrogen bonds
Basic R-groups contain ______ atoms
nitrogen and hydrogen