C1.1 Enzymes & Metabolism
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Define catalyst
A substance that speeds up chemical reactions without being used up or chemically altered
Define enzyme
A biological catalyst synthesised by living cells
Define activation energy
Minimum amount of energy needed to start a chemical reaction
Describe the role of enzymes, & how they carry it out
Enzymes increase the rate of a specific biochemical reaction, that would otherwise be too slow to sustain life
They do this by:
Converting substrates into products
Lowering the activation energy → reactions occur more easily
Define metabolism
The sum of all chemical reactions in a living organism
What are the two types of metabolic reactions?
Catabolic reactions - Involves the breaking down of molecules (e.g. respiration)
Anabolic reactions - Involves building molecules (e.g. protein synthesis)
Describe how enzymes control reactions (5)
Each reaction catalysed by a specific enzyme as their active site only fits certain substrates (enzyme specificity)
These enzymes regulate the reaction speed & direction:
Speed up/slow down parts of metabolism
Switch pathways on/off depending on conditions (e.g. exercise vs fasting)
Enzyme-controlled reactions form chains/cycles → product of one reaction becomes substrate for next
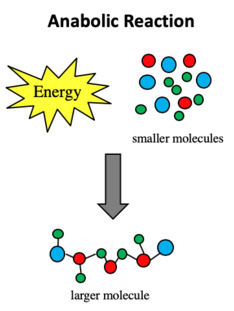
Describe anabolic reactions, & give examples (7)
Function: Builds larger molecules from smaller ones
Monomers + Energy → Macromolecule + Water
——————————————————————————————————
Energy: Requires energy (endergonic)
——————————————————————————————————
Examples:
Condensation reactions where water is removed to join monomers & form macromolecules
Protein synthesis - joins amino acids to make polypeptides
Glycogen formation - joins glucose units for energy storage in animals
Photosynthesis - uses CO2 and H2O to build glucose
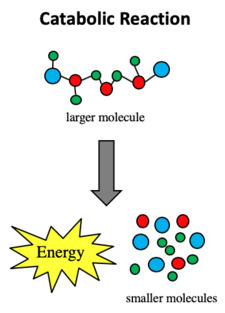
Describe catabolic reactions, & give examples (7)
Function: Breaks down larger molecules into smaller ones
Macromolecule + Water → Monomers + Energy
——————————————————————————————————
Energy: Releases energy (exergonic)
——————————————————————————————————
Example:
Hydrolysis reactions where water is added to break bonds & provide raw materials/energy for cells
Digestion - breaks down proteins, carbs, fats, into monomers (e.g. amino acids, sugars, fatty acids)
Respiration - glucose is broken down to release ATP
What type of proteins are enzymes?
Globular proteins → compact & water-soluble nature
Explain what controls enzyme shape & its influence on catalysis (7)
Their specific 3D conformation (tertiary structure) is formed by interactions between amino acids:
Hydrogen bonds
Ionic bonds
Disulphide bridges
Hydrophobic interactions
——————————————————————————————————
Significance to catalysis:
These interactions determine precise shape & chemical properties of the active site
This maintains its function: bind substrate temporarily & lower activation energy
Enables chemical reaction to proceed faster w/o enzyme being depleted
What is the Induced-Fit model?
A revised model that suggests enzymes & substrates change shape slightly when they bind
This disproves the lock-and-key model that proposed a perfect fit
Describe the stages of Induced Fit
Approach - Substrate enter the enzyme’s active site
Conformational change - The enzyme’s active site molds itself around the substrate. The substrate may also change shape to fit more snugly.
Catalysis - It puts strain on substrate bonds, making them easier to break/rearrange during the reaction
Release - After the reaction, products have different shapes & chemical properties, causing detachment from the active site. The enzyme reverts to original conformation, ready to bind another substrate.
Why is induced fit significant?
1) Specificity - Ensures only the correct substrate can trigger conformational changes
2) Efficiency - Weakening bonds lower activation energy for reactions
Explain the role of molecular motion (3)
Enzymes & substrates are constantly moving randomly in a fluid environment, allowing collisions to occur
Collisions must occur in the right orientation & with enough energy
A successful collision between the active site & substrate forms an enzyme–substrate complex
Describe factors affecting enzyme collisions (3)
1) Temperature - As temperature increases, molecules gain kinetic energy & move quicker = more frequent collisions
2) Concentration - Higher enzyme/substrate concentration = higher chance of collisions
3) Molecular size - Smaller molecules move faster = more frequent collisions
What happens to enzymes & substrates when movement is restricted?
Sometimes, movement is restricted but reactions still occur:
Immobilised substrates - Large molecules like DNA may be anchored in place so enzymes must move to them
Immobilized enzymes - Some enzymes are fixed to membranes so substrate must move to reach the enzyme’s active site
Define denaturation
Process by which an enzyme’s three-dimensional shape is permanently altered, leading to a loss of function
What are the causes of denaturation?
1) High temperatures
2) Extreme pH
3) Chemical agents (e.g. alcohol or heavy metals)
→ all disrupts/breaks bonds maintaining conformation
What are the effects of denaturation?
Active site changes shape
Substrate can’t bind properly
Enzyme loses activity, often irreversibly → cannot lower activation energy & catalyse reaction
How do active sites maintain enzyme-substrate specificity?
Each active site has:
1) Shape that matches one substrate
2) Amino acids w/ specific chemical properties (e.g. charge, hydrophobicity)
This allows precise interactions which maintain enzyme-substrate specificity
Hence, enzymes will only catalyse one reaction / group of closely-related reactions
State factors rate of an enzyme-catalysed reaction depends on
1) How frequent enzyme & substrate molecules collide (collision theory)
2) Whether the enzyme’s active site remains functional (not denatured)
Describe the effect of temperature on enzyme activity
Temperature | Effect |
Increasing temp | Molecules move faster, increasing collision frequency → faster reaction |
Optimum temp | Temperature where enzyme works most efficiently |
Too hot |
|
Describe the effect of pH on enzyme activity
pH | Effect |
Optimum pH | pH where enzyme works most efficiently (often pH 7 for human enzymes) |
Extreme pH | Too acidic/basic
|
Describe the effect of substrate concentration on enzyme activity
Temperature | Effect |
Low substrate | Fewer collisions → slower reaction |
Increasing substrate | More frequent collisions → faster reaction |
High substrate (enzyme saturation) | All active sites are occupied — reaction rate levels off |
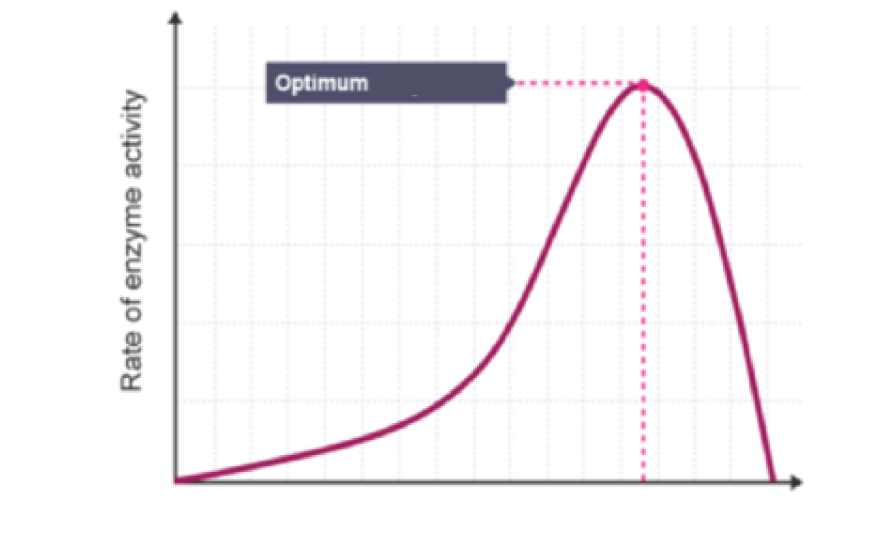
What does this graph represent? Describe its trend
The effect of temperature on enzyme activity:
Rises steeply
Peaks at optimum
Drops sharply due to denaturation
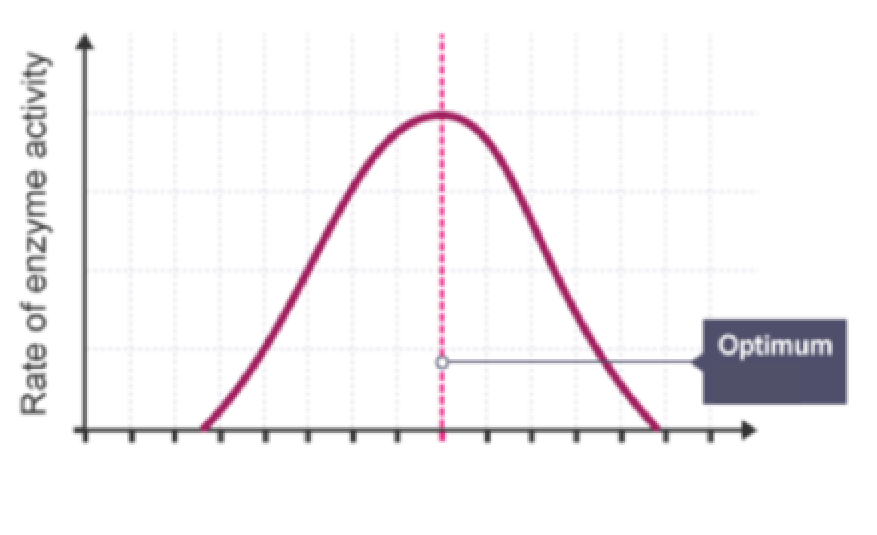
What does this graph represent? Describe its trend
The effect of pH on enzyme activity:
Rises steadily
Peaks at optimum
Drops steadily
What does this graph represent? Describe its trend
The effect of substrate concentration on enzyme activity:
Rapid increase
Plateaus
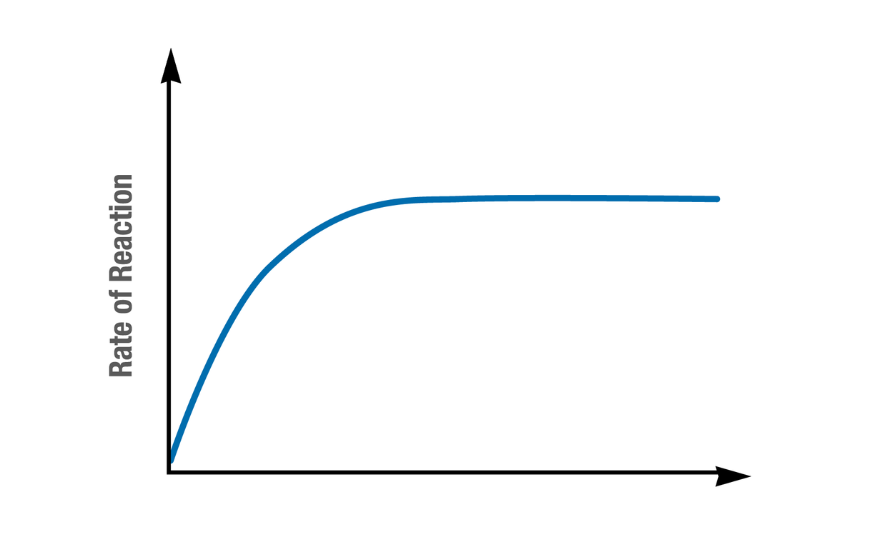
What factors is enzyme activity measured by?
1) Decrease in substrate concentration
2) Increase in product concentration
What is the formula of rate of reaction?
Rate of reaction = Change in amount (substrate / product) ÷ Time taken
Give examples of enzyme-catalysed reactions & their measurement methods
Enzyme | Reaction | Measurement Method |
Catalase | Hydrogen peroxide → Water + Oxygen | Measure volume of O₂ gas released |
Amylase | Starch → Maltose | Iodine test: Stays orange-brown, not turning blue-black = starch digested |
Lipase | Lipid → Fatty acids | pH indicator: solution becomes more acidic |
How is the initial rate calculated?
1) Draw a tangent at steepest part of graph
2) Calculate gradient (product ÷ time taken)
Explain how enzymes change activation energy to catalyse reactions
Enzymes lower activation energy (Ea) → reactions happen faster & more easily:
Energy is used to break bonds in substrates (endergonic) to allow reaction to begin
Once reaction is complete, energy is released to form bonds in products (exergonic)

Where can enzyme-catalysed reactions occur?
Inside cells = intracellular
Outside cells = extracellular
Describe intracellular enzyme reactions, & give examples
These happen within the cell’s cytoplasm or organelles
Examples:
Process | Location | Function |
Glycolysis | Cytoplasm | Breaks glucose into pyruvate (first stage of respiration) |
Krebs Cycle | Mitochondrial matrix | Produces ATP, CO₂, & other products in aerobic respiration |
Describe extracellular enzyme reactions, & give an example
These happen outside the cell, usually in the digestive system
Example: Chemical digestion in the gut
Enzyme | Site of Action | Function |
Amylase | Mouth & small intestine | Breaks down starch into maltose |
Protease (e.g. pepsin, trypsin) | Stomach, small intestine | Breaks down proteins into amino acids |
Lipase | Small intestine | Breaks down lipids into fatty acids & glycerol |
Explain heat generation in metabolic reactions
Metabolic reactions are never 100% efficient
Some energy from glucose, fats, & proteins is transferred to ATP
Inevitably, the rest is lost as heat energy (byproduct)
Explain the importance of heat
Thermoregulation in endotherms (warm-blooded animals)
Mammals & birds rely on metabolic heat to:
1) Maintain a constant internal body temperature
2) Function in cold environments
3) Support enzymatic reactions, which work best at optimum temperature
Define metabolic pathway
A series of enzyme-catalysed chemical reactions in a cell
What are the types of metabolic pathways?
1) Linear
2) Cyclical
Describe linear pathways, & give an example (5)
Straightforward process: substrates are converted into a final product via a series of steps, each catalysed by enzymes
The process does not loop back
Example: Glycolysis
Occurs in cytoplasm
Breaks glucose into two pyruvate molecules & net gain of 2 ATP + 2 NADH
Describe cyclical pathways, & give examples (8)
End products are fed back into first step → regenerating initial substrates
Continuous cycle as long as substrates & enzymes are available
Examples:
1) Krebs Cycle
Occurs in mitochondrial matrix
Oxidising acetyl-CoA to synthesise ATP, NADH, FADH2, CO2
2) Calvin Cycle
Occurs in chloroplast stroma
Uses CO2, ATP, NADPH to build glucose in photosynthesis
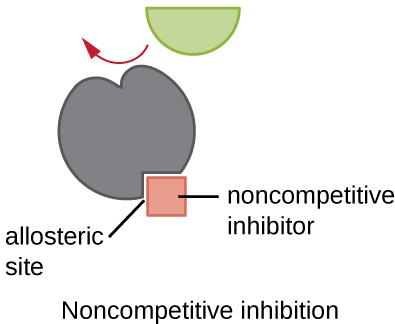
Define allosteric site
Any region on an enzyme that is not the active site
Describe the process of non-competitive inhibition
Specific molecules (called allosteric effectors) can bind to allosteric sites, usually non-competitive inhibitors
Binding causes a conformational change in the enzyme
This alters the active site’s shape → preventing substrate binding
Even if substrate concentration increases, inhibitor’s effect remains → reducing/blocking enzyme catalysis
⭐ This binding is typically reversible
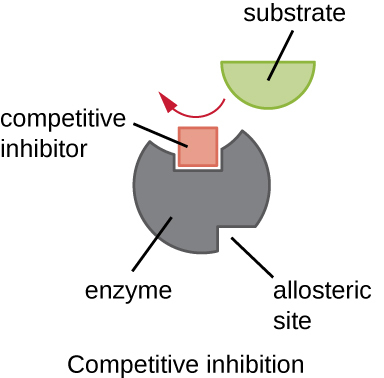
Describe the process of competitive inhibition
Molecule w/ a similar shape to substrate fits into enzyme’s active site
While the inhibitor is bound, the real substrate cannot enter
No product is formed while the inhibitor occupies the site
⭐ The inhibition is reversible → if inhibitor detaches, the substrate has another chance
Give an example of competitive inhibition
Statins
Drugs that competitively inhibit the enzyme HMG-CoA reductase, by blocking substrate binding
Involved in cholesterol synthesis → reduces cholesterol levels in human body
Describe the differences between competitive & non-competitive inhibition
Feature | Competitive | Non-competitive |
Inhibitor binding site | Active site | Allosteric site |
Substrate competes? | ✅ | ❌ |
Effect of high substrate concentration | Can overcome inhibition | Cannot overcome |
Effect of low substrate concentration | Reduced rate of reaction | |
What is feedback inhibition?
A type of negative feedback used to regulate metabolic pathways
Describe the process of feedback inhibition
In a metabolic pathway, when enough of the end product is made, it binds to an enzyme that catalyzes an earlier step
This inhibits the enzyme’s activity, slowing or stopping the entire pathway
When the end product is used up, the inhibition lifts, & the pathway resumes
Describe an example of feedback inhibition (7)
Example: Isoleucine Biosynthesis
Substrate: Threonine
Product: Isoleucine
Isoleucine is an amino acid produced from threonine in a five-step metabolic pathway
When isoleucine builds up, it binds allosterically to the pathway’s first enzyme: threonine deaminase
This changes the enzyme’s shape, preventing threonine from binding to it
As a result, no more isoleucine is made until the level drops again
Describe the strengths of feedback inhibition (3)
1) Prevents waste of resources and energy
2) Maintains homeostasis in the cell
3) Ensures balanced production of essential molecules
Describe the process of mechanism-based inhibition
An inhibitor binds to an enzyme’s active site
A chemical reaction takes place, permanently altering the enzyme
This causes the enzyme to be irreversibly inactivated & lose function
Give an example of mechanism-based inhibition, & how it works (6)
Penicillin
Penicillin targets transpeptidase enzymes in bacteria
These enzymes help cross-link peptidoglycan → a key component in bacterial cell walls
How penicillin works:
Penicillin resembles the enzyme’s natural substrate, binding to the active site of transpeptidase
A chemical reaction forms a covalent bond with the enzyme
This permanently disables the enzyme, causing no cell wall formation
Due to lack of structural support, the bacterial cell bursts
How is resistance to penicillin achieved? (2)
Some bacteria evolve by altering their transpeptidase enzyme
These altered enzymes (called penicillin-binding proteins) no longer allow penicillin to bind