The particle model
1/11
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
Definition of density?
density describes how closely packed the particles are in a solid, liquid or gas.
how are the particles arranged in a solid?
particles are tightly packed in a regular structure
how are the particles arranged in a liquid?
particles are tightly packed but free to move past each other.
how are the particles arranged in a gas?
particles are spread out and move randomly.
calculation for density?
density (ρ) is measured in kilograms per cubic metre (kg/m3 )
mass (m) is measured in kilograms (kg)
volume (V) is measured in cubic metres (m3)
method for measuring specific heat capacity of water experiment (core prac)
Place one litre (1 kg) of water in the calorimeter.
Place the immersion heater into the central hole at the top of the calorimeter.
Clamp the thermometer into the smaller hole with the stirrer next to it.
Fully insulate the calorimeter by wrapping it loosely with cotton wool.
Record the temperature of the water.
Connect the heater to the power supply and a joulemeter and turn it on for ten minutes. Stir the water regularly.
After ten minutes the temperature will still rise even though the heater has been turned off and then it will begin to cool. Record the highest temperature that it reaches and calculate the temperature rise during the experiment.
what are some hazards of measuring heat capacity of water?
hazard: hot immersion heater and sample material causing burnt skin preventing: (Do not touch when switched on. Position away from the edge of the desk. Allow time to cool before packing away equipment. Run any burn under cold water for at least 10 minutes.)
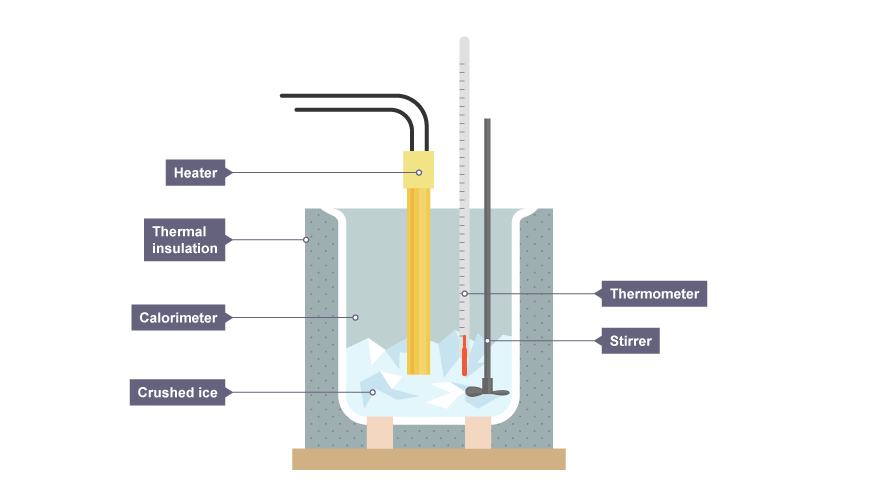
aim of measuring temperature of melting ice?
To obtain a graph showing changes in temperature over time for heated ice.
method of measuring the temp of melting ice
Place 50 grams of crushed ice straight from the freezer into the calorimeter.
Place the immersion heater into the central hole at the top of the calorimeter.
Clamp the thermometer with its bulb in the ice but near the top of the ice.
Record the temperature of the ice.
Connect the heater to the power supply and joulemeter, turn it on and record the temperature every 20 seconds.
Continue until the thermometer bulb is no longer under the level of the water.
hazards of measuring the temp of melting ice?
Hot immersion heater and water ways it could be hazardous is burning your skin preventing:(Do not touch when switched on. Position away from the edge of the desk. Allow time to cool before packing away equipment. Run any burn under cold water for at least 10 minutes.)
one method of calculating density is?
Method one
Use a ruler to measure the length (l), width (w) and height (h) of a steel cube.
Place the metal cube on the top pan balance and measure its mass.
Calculate the volume of the cube using (l × w × h).
Use the measurements to calculate the density of the metal.
Use vernier callipers to measure the diameter of the sphere.
Place the metal sphere on the top pan balance and measure its mass.
Calculate the volume of the sphere using 43π(d2)3
Use the measurements to calculate the density of the metal.
method two
Place the stone on the top pan balance and measure its mass.
Fill the displacement can until the water is level with the bottom of the pipe.
Place a measuring cylinder under the pipe ready to collect the displaced water.
Carefully drop the stone into the can and wait until no more water runs into the cylinder.
Measure the volume of the displaced water.
Use the measurements to calculate the density of the stone.
method three:
Place the measuring cylinder on the top pan balance and measure its mass.
Pour 50 cm3 of water into the measuring cylinder and measure its new mass.
Subtract the mass in step 1 from the mass in step 2. This is the mass of 50 cm3 of water.
Use the measurements to calculate the density of the water.
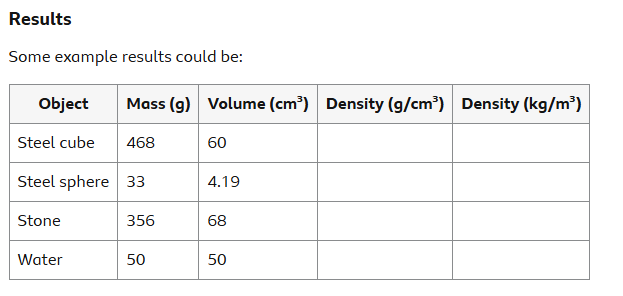
Analysis of how to calculate density
Using those results, the densities can be calculated using:
Density = mass ÷ volume
Mass of steel cube = 468 g
Volume of steel cube = 60 cm3
Density = mass ÷ volume = 468 ÷ 60 = 7.8 g/cm3 (= 7,800 kg/m3 )
Diameter of steels sphere = 2 cm
Mass of steel sphere = 33 g
Volume of steel sphere = 43π(d2)3 = 4.19 cm3
Density = mass ÷ volume = 33 ÷ 4.19 = 7.9 g/cm3 (= 7,900 kg/m3 )
For a stone of mass 356 g, the volume of water displaced into the measuring cylinder is 68 cm3
Density = mass ÷ volume = 356 ÷ 68 = 5.2 g/cm3 (= 5,200 kg/m3 )
Mass of 50 cm3 of water is found to be 50 g. Density = mass ÷ volume = 50 ÷ 50 = 1 g/cm3 (= 1,000 kg/m3 )