Isotopes and radioisotopes
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What's the atomic mass?
Average of the sum of all protons and neutrons of that element
What is the atomic number?
the number of protons in an atom
What is mass number?
sum of protons and neutrons
What is an isotope?
Atoms of the same element with different numbers of neutrons, resulting in different mass numbers.
How do you write the nuclide symbol of carbon?
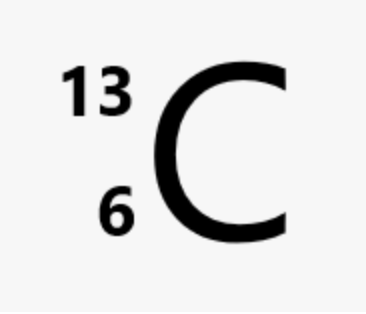
What are radioisotopes?
Isotopes that are unstable and emit radiation as they decay into more stable forms.
What did john Dalton find?
John Dalton found out about atoms and thought they were really small particles that can’t be made any smaller.
What did Thomson discover?
He discovered the plum pudding model
What did rutherford discover?
He discovered the nucleus of the atom and the concept of radioactive decay through his gold foil experiment.
What did bohr discover?
Bohr discovered that electrons orbit the nucleus in specific energy levels, leading to the Bohr model of the atom. Planetary model.
What did chadwick discover?
Neutrons
What are the three forms of radioactive decay?
Alpha decay, beta decay, and gamma decay.
Describe Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, consisting of two protons and two neutrons, resulting in a new element with a lower atomic number.
Describe beta decay
Emits an electron
Describe gamma decay
Gamma decay is a type of radioactive decay in which an unstable atomic nucleus releases energy in the form of gamma rays, resulting in no change in atomic number or mass number.
What can alpha decay go through?
Alpha decay can penetrate a few centimeters in air and can be stopped by paper or skin.
What can beta decay go through?
Beta decay can penetrate further than alpha particles, typically several meters in air, and can be stopped by a few millimeters of plastic or glass.
What can a gamma decay penetrate?
Gamma decay can penetrate most materials, including several centimeters of lead or meters of concrete, making it highly penetrating.
What is a radioisotope half-life?
The time required for half of the radioactive atoms in a sample to decay into a more stable form. It is a characteristic property of each radioisotope.
What would make an atom unstable?
An atom becomes unstable when it has too many or too few neutrons compared to protons, typically leading to radioactive decay.
How many protons would make an element unstable?
82 protons
How to find the most stable atom of an element?
If it’s mass number is the closest to the mass number on the periodic table.
What happens every half life?
The number of radioactive atoms halve.
What do you measure the number of decays per second of a radioactive sample?
Becquerels (Bq)
What is the maximum amount of electrons in the first shell?
2 electrons
What is the maximum in the second shell?
8
What is the maximum in the third shell?
8
What is the maximum in the fourth shell?
Whatever is left
Why do different elements have different flame colours?
Because of their electron configuration
What does the last number of the group number show?
The amount of valence electrons
What are valence electrons?
The amount of electrons in the last shell
What are the qualities of noble gases?
gaseous at room temperature and never reacts
Properties of metals
solid at room temperature
shiny
conducts electricity and heat
can be reactive chemically
properties of non metals
dull
insulator: doesn’t conduct electricity or heat
can be reactive
When do you know an element is a metal?
it loses one electron
what do you call an atom that loses one or more electrons, (fluorine)
fluoride ion
What is a metalloid?
A chemical element that has some metal and non-metal properties.
how do you know how many shells an element has?
What period it’s in
how do metal atoms form ions?
they lose electrons to form positive ions
What is an anion?
it is a negatively charged atom
What is a cation?
A positively charged ion