kidney cap
1/376
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
377 Terms
functions of the kidneys
Regulate the volume and composition of the extracellular fluid (ECF)
Eliminate potentially toxic metabolic wastes and foreign compounds
Maintain water balance in body
Maintaining proper plasma volume (BP)
Maintain osmolarity (solute concentration) of body fluids
Regulating quantity and concentration of extracellular fluid ions
Maintaining acid-base balance
convert vitamin D to active form
Producing erythropoietin
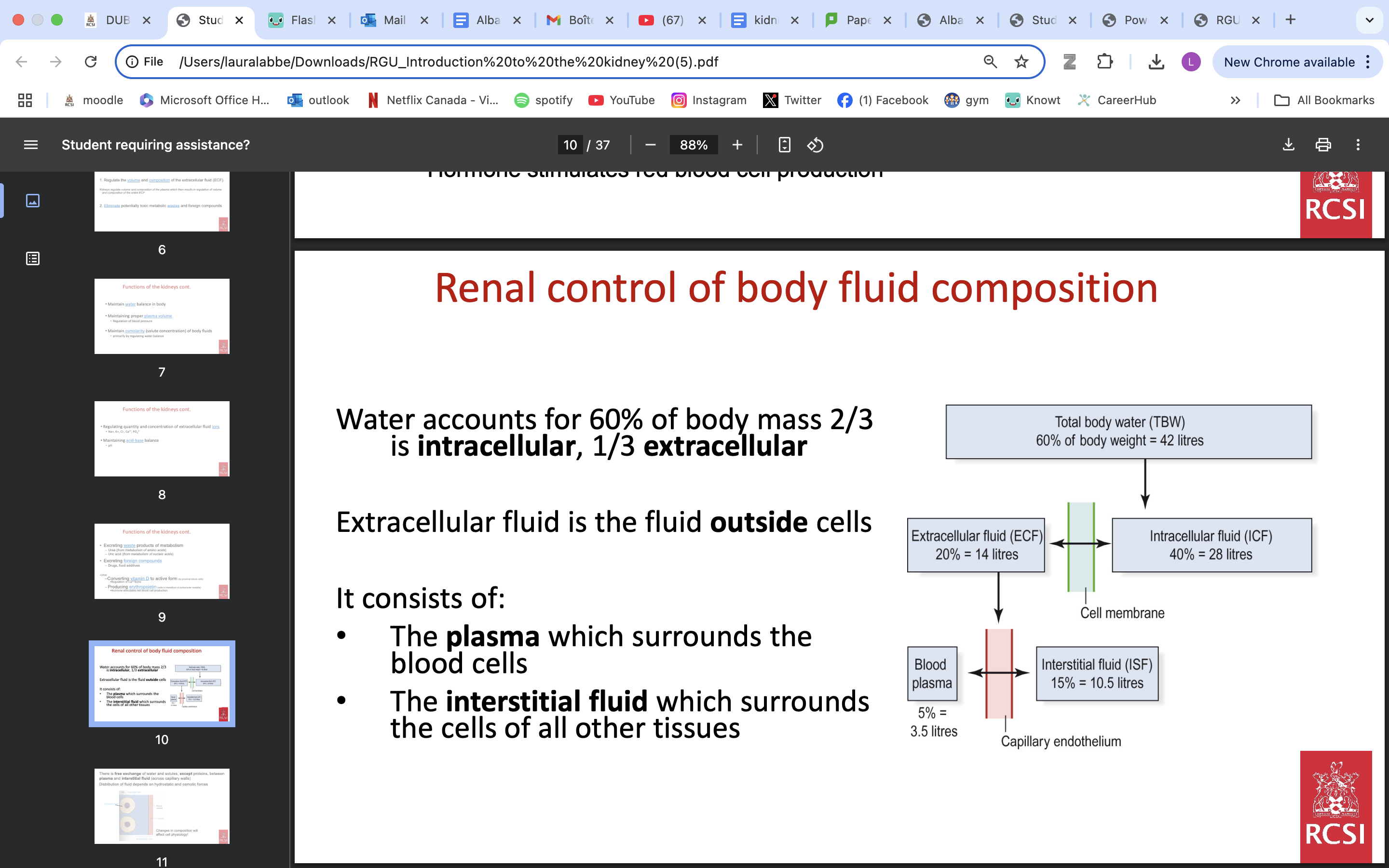
renal control body fluid composition
A decrease in water content, by increasing the NaCl concentration, increases osmolarity
An increase in water content, by lowering the NaCl concentration, lowers osmolarity
The osmolarity of plasma and ECF is normally maintained constant at 283 ± 11 mosmol/l.
Basic processes performed by the nephron
1) Filtration of blood
2) Tubular reabsorption
3) Tubular secretion
Renal blood flow (RBF)
• First process in nephron is ultrafiltration of the plasma in the glomerulus. This step is dependent on generation of strong hydrostatic pressure in nephron
• Kidney requires abundant blood supply
• Blood supply to the two kidneys is via the renal arteries
• Blood flow to the kidneys is ~1.2 L/min.
1/5 of cardiac output*
blood supply to nephron
Renal artery subdivides to form many afferent arterioles which each supply a nephron
Glomerular capillaries recombine to leave Bowmans Capsule as efferent arterioles.
Efferent arterioles give rise to peritubular capillaries which invest the tubular system of each nephron.
These then recombine to form venules and the renal vein
Function of Peritubular Capillaries
1. Nutritive
2. Reabsorptive
3. Secretive
What passes through the filtration barrier to form the tubular filtrate?
All components of plasma, except cells and proteins, pass
(a small amount of protein does pass but is immediately reabsorbed – a normal sample of urine should have no protein).
So, useful substances as well as waste pass through
substances reabsorbed during active tubular resorbption
glucose
amino acids
Na+
substances reabsorbed during passive tubular resorbption
water
chloride ion
Volume of tubular filtrate formed
Approx. 180 litres of filtrate forms per day - entire ECF “treated” ~10 times per day
Approx. 1.5 litres of urine are excreted
Therefore, approx. 178.5 litres of filtrate must be reabsorbed (returned to the blood) per day
Distribution of Renal Blood Flow (RBF)
Blood flow to the cortex: 90%
Blood flow to medulla: 10%
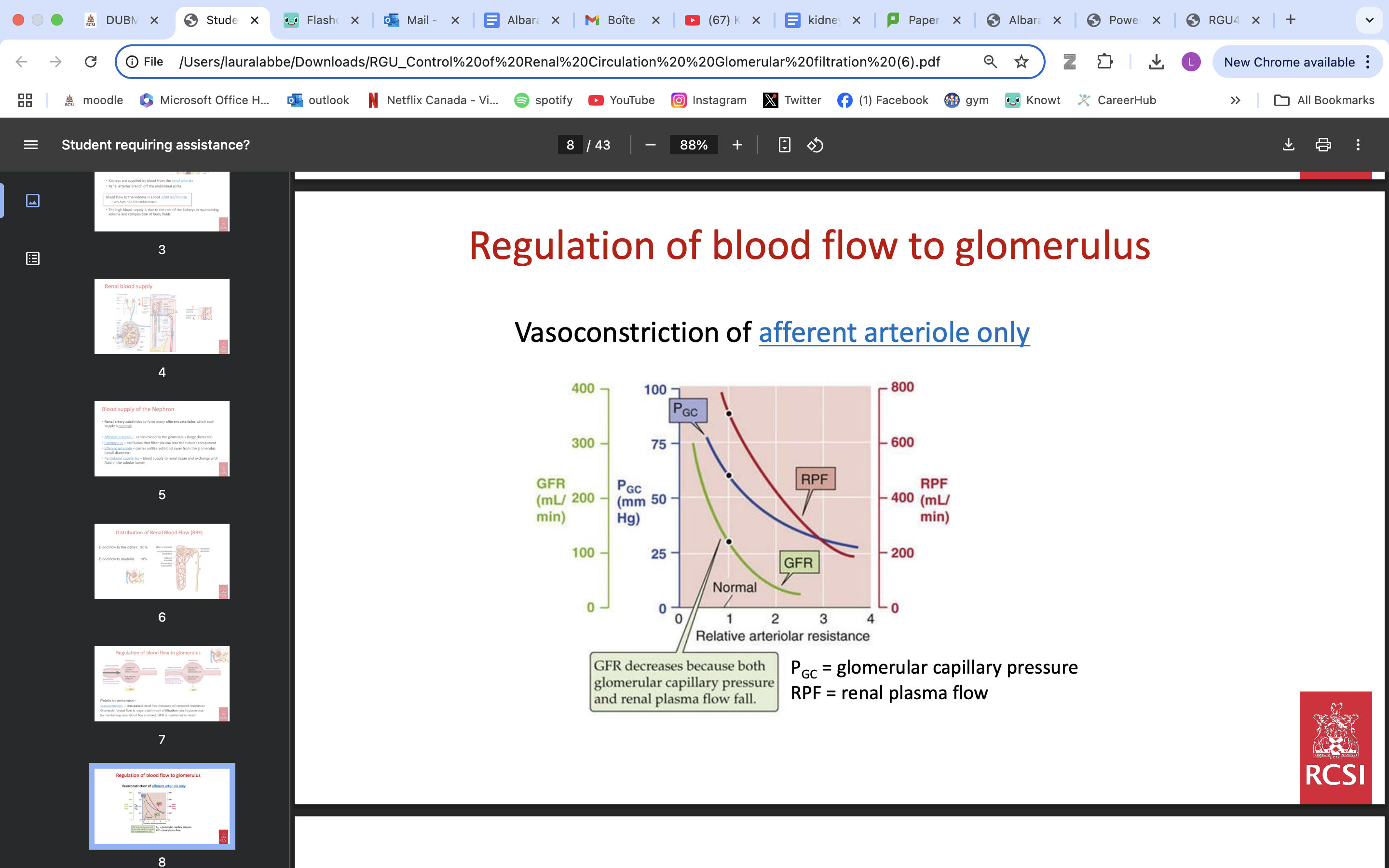
Regulation of blood flow to glomerulus
vasoconstriction → decreased blood flow (because of increased resistance)
Glomerular blood flow is major determinant of filtration rate in glomerulus
By maintaining renal blood flow constant, GFR is maintained constant
VIA AFFERENT ARTERIOLES VASOCONSTRICTION
Two mechanisms are responsible for the autoregulation of RBF and GFR
1. Myogenic Mechanism: Responds to arterial pressure changes
as pressure ↑ so resistance ↑ and therefore flow remains constant.
2.Tubuloglomerular Feedback Mechanism: Responds to [Na Cl]
when GFR increase→ NaCl concentration rise in LoH→ macula densa release adenosine→ increased resistance
RBF and GFR maintained if MAP between 80-180mmhg
![<p><span style="color: red"><strong>1. Myogenic Mechanism</strong></span><strong>: Responds to arterial pressure changes </strong></p><ul><li><p>as pressure ↑ so resistance ↑ and therefore flow remains constant.</p></li></ul><p><strong>2.Tubuloglomerular Feedback Mechanism: Responds to [Na Cl]</strong></p><ul><li><p>when GFR increase→ NaCl concentration rise in LoH→ macula densa release adenosine→ increased resistance </p></li></ul><p></p><p>RBF and GFR maintained if MAP between 80-180mmhg</p>](https://knowt-user-attachments.s3.amazonaws.com/42fbc40e-442a-4815-987f-769131e19888.png)
Benefits of autoregulating RBF
Stabilises the amount of filtered solutes that reach the tubules over a wide range of arterial pressures
Protects fragile glomerular capillaries against increases in perfusion pressure
allows consistency vs light exercise, changes in posture etc…
under what conditions do RBF and GFR change
sympathetic nervous system activity→ vasoconstriction
adrenaline and angiotension 2→ vasoconstriction
bradykinin→ vasodilation
The role of extrinsic nervous and hormonal influences in regulation of RBF & GFR;
A person at rest with normal circulating blood volume
Activity in SNS fibres supplying renal vessels is zero.
RBF and GFR maintained constant by auto-regulation
The role of extrinsic nervous and hormonal influences in regulation of RBF & GFR;
Effect of severe exercise, pain, severe emotional stimuli on RBF & GFR
Sympathetic NS activity in renal vessels may be detected and adrenaline is released from adrenal medulla
effects: RBF and GFR decreased.
The role of extrinsic nervous and hormonal influences in regulation of RBF & GFR;
Effect of crisis situation e.g haemorrhage on RBF & GFR
High degree of sympathetic NS activity may be detected in renal vessels. Also, adrenaline is released and circulating levels of angiotensin 2 increase
RBF and GFR decreased.
major force driving glomerular filtration
Glomerular capillary blood pressure
Large diameter of afferent vs efferent arteriole generates pressure pushing out plasma through (leaky) capillaries
what influences the passage of components through the filtration barrier to form the tubular filtrate
• Molecular size
• Charge (negatively charged molecules are restricted by barrier)
• Molecule shape – deformable molecules pass better than rigid ones
average GFR
Defined as: the millilitres (mL) of plasma filtered per minute through all glomeruli (i.e. both kidneys)
In adult males, GFR = 125 ml/min
In adult females, GFR = 115 ml/min
Forces favouring Glomerular Filtration
Glomerular capillary blood pressure
net filtration pressure
Forces working against Glomerular Filtration
Plasma-colloid osmotic pressure
bowman’s capsule hydrostatic pressure
unregulated influences on GFR examples
Decrease in plasma protein reduces force opposing filtration so this increases GFR (burn patients)
Urinary tract blockage – increases pressure in Bowman’s capsule – decreases GFR
Plasma-colloid pressure increases in dehydrating diarrhoea so GFR is decreased
Steps of trans-epithelial transport - tubular resorption
1) Substance leaves tubular fluid
2) Passes through cytosol of tubular cell
3) Crosses basolateral membrane
4) Diffuses through interstitial space
5) Penetrates capillary wall to enter blood plasma
FLUID MOVEMENT ALONG THE NEPHRON OCCURS
via osmosis
Secretion and absorption of solutes across the tubular epithelium creates osmotic gradients
epithelium in tubules act as semi permeable membrane
what is tubular reabsorption
process by which solutes and water are removed from the tubular fluid and transported into the blood
include electrolytes, glucose, proteins, amino acids, urea, etc
Finely tuned process that maintains homeostasis of blood volume, pressure, pH and osmolarity
can be active or passive
Filtrate osmolarity changes dramatically throughout the nephron
filtrate osmolarity PCT
300 mOsm/L
filtrate osmolarity descending LoH
1200 mOsm/L as water is reabsorbed
what is tubular secretion
transport of solutes from peritubular capillaries/interstitium into the tubular lumen
differs from reabsorption as it deals with clearing substances from the blood, rather than retaining them
Secreted substances are typically waste/unwanted products (e.g. K+ , H+ , NH4 + , Creatinine, Urea, & some hormones & drugs)
can be passive or active
epithelium PCT
cuboidal epithelium
microvilli increase surface area for reabsorption
mitochondria ensure energy is available for active transport needed for efficient reabsorption.
epithelium thick LoH
cuboidal
epithelium thin LoH
squamous
Two common properties of tubular epithelial cells allow them to carry out their absorptive & secretory functions
tight junctions
functional polarity
tight junctions
allow cells to carry out their absorptive & secretory functions
Point of contact between neighbouring cells
Consist of transmembrane proteins that form homotypic bonds with neighbouring cells.
Are permeable to water and ions/small molecules.
forms paracellular pathway
Structural components = occludins, claudins, and junctional adhesion molecule (JAM)
functional polarity
allow cells to carry out their absorptive & secretory functions
Ability of epithelial cells to express different transport proteins on their apical and basolateral sides - enables vectorial transport of solutes
3 main types of epithelial transport protein renal tubules
i) ATPase pumps
ii) Channels
iii) Carriers: Co-transporters and Exchangers
Water crosses cell membranes by two routes
• through the TJs and paracellular space
• through water channels – Aquaporins
transport is bidirectional, in accordance with prevailing osmotic gradients established by active solute transport
where is aquaporin 1 found
proximal tubule and descending thin limb
where is aquaporin 3 and 4 found
expressed in the DCT/Collecting Ducts
Important in regulation of water reabsorption by antidiuretic hormone (ADH).
proximal covoluted tubule
Many filtered solutes are reabsorbed in the early proximal convoluted tubule
(e.g., NaCl and other ions, glucose, amino acids, phosphate, lactate, citrate, urea, etc).
Na reabsorbed early- as most other things
Cl reabsorbed late
Much of the uptake occurs through Na+ /Nutrient Cotransporters
Na+ /glucose cotransporter
Na+ /amino acid Cotransporter
Water is reabsorbed passively due to osmosis (70% water resorption occurs here)
Glycosuria
Normally, almost 100 percent of glucose is reabsorbed in the PCT.
Increases in filtered load may lead to some glucose being excreted in the urine = Glycosuria
detectable via dipstick test
causes
DM
pregnancy
↑ renal blood flow results in ↑ glucose being filtered
falconi syndrome
Glomerular filtration of protein is dependent primarily on
Molecular size - Low MW proteins (15 000-40 000 kDa) readily filtered
ionic charge- Extracellular matrix within the basement membrane of the filtration barrier contains negatively charged proteins and therefore repels negatively charged proteins in the plasma
Molecular shape -Deformable molecules can pass through more readily than rigid ones
Plasma Concentrations - Elevated plasma levels of a protein lead to increased filtration
Normally, small peptide hormones and albumin (~ 200 g) are filtered
steps Protein reabsorption by PCT
filtered proteins bind to receptors on luminal membrane - megalin & cubulin
bound proteins undergo endocytosis
endosomes fuse with lysosomes
lysosome proteases degrade proteins
resulting amino acids exit via basolateral transporters
proteinuria
defined as increased amounts of protein in urine
Diagnosed as foamy urine and dipstick test
causes
glomerular damage
alterations in tubular reabsorption (e.g., Fanconi syndrome)
Increased plasma concentration of low MW proteins
PCT and organic ions
- Organic ions - typically xenobiotics, such as drugs or environmental/dietary chemicals, and some endogenous metabolites.
secreted in PCT
Renal elimination of organic ions has great practical significance
prevent exposure to potentially dangerous xenobiotics
limit the efficacy of some drugs.
fanconi syndrome
a disease that is associated with dysfunction of the proximal tubule of the kidney.
characterized by the loss of, amino acids, protein, glucose and bicarbonate in the urine
Symptoms: thirst, polyuria, vomiting. failure to thrive, frailty, bone pain, rickets, acidosis
causes; hereditary/ exposure to chemicals or drugs
Treatment: Hydration and supplementation of lost substances (e.g. HCO3, phosphate & vitamin D
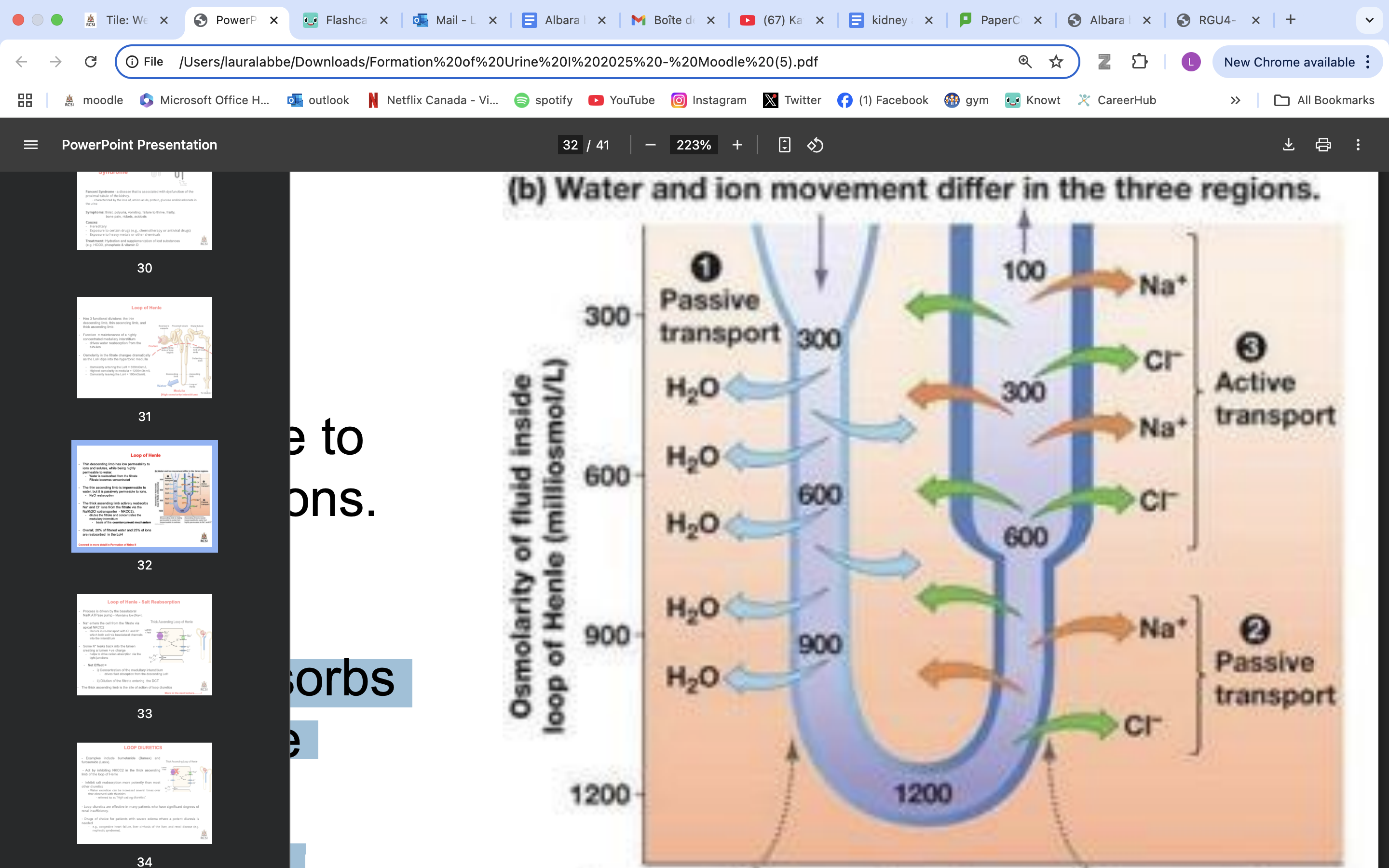
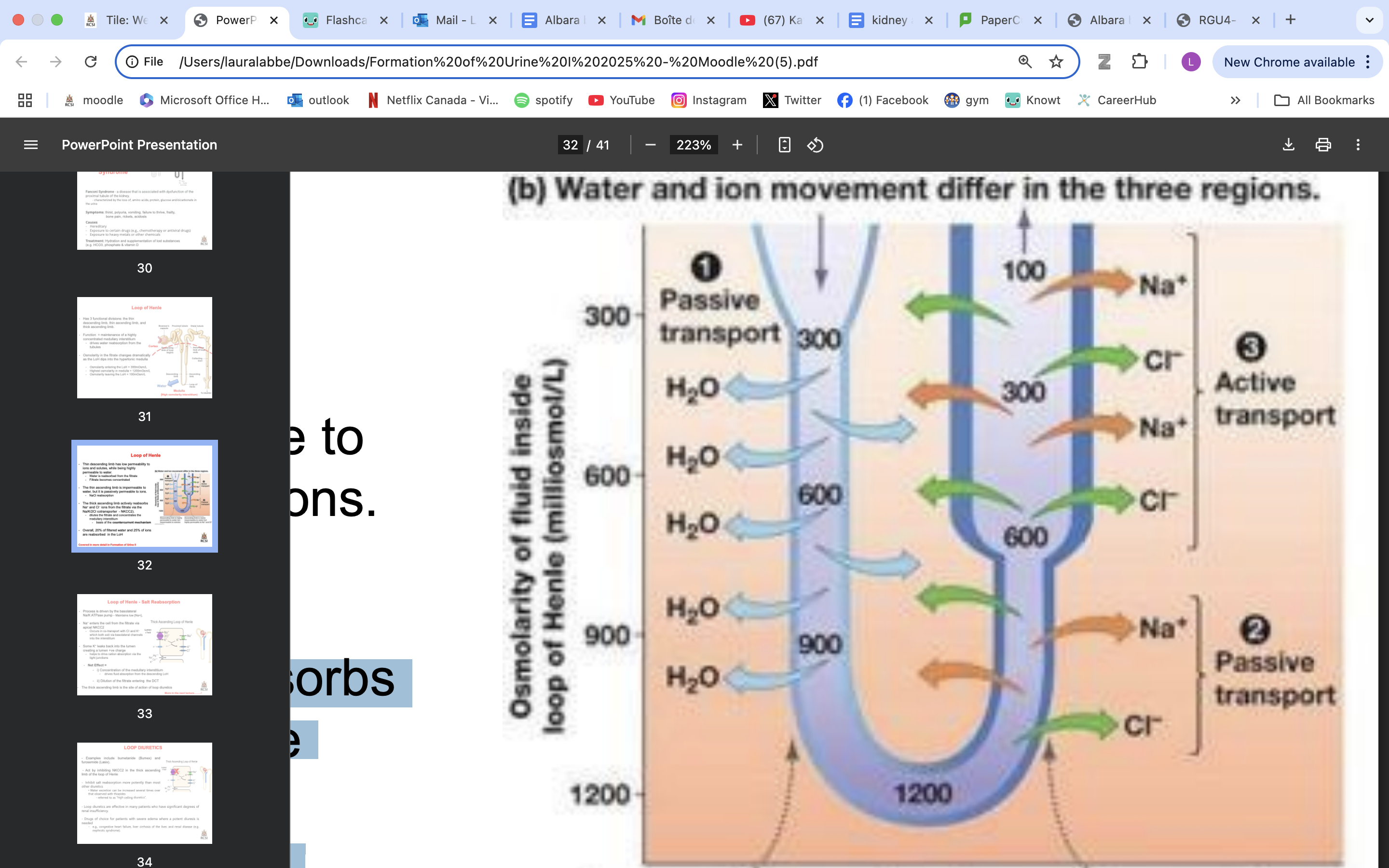
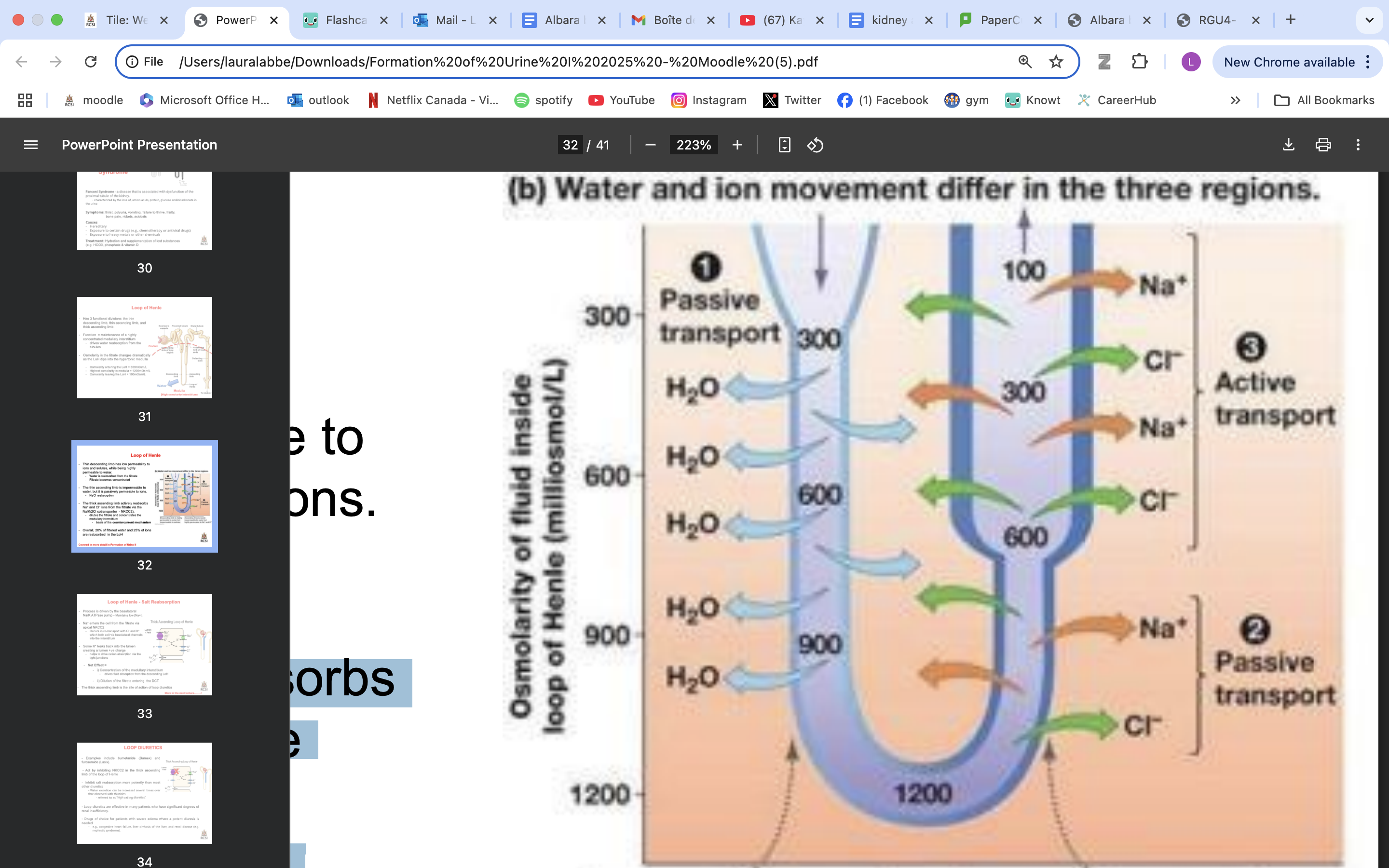
LoH functional divisions
the thin descending limb
thin ascending limb
thick ascending limb.
LoH function
maintenance of a highly concentrated medullary interstitium
drives water reabsorption from the tubules
Osmolarity in the fitrate changes dramatically as the LoH dips into the hypertonic medulla
Osmolarity entering the LoH = 300mOsm/L
Highest osmolarity in medulla = 1200mOsm/L
Osmolarity leaving the LoH = 100mOsm/L
thin descending LoH role
has low permeability to ions and solutes, while being highly permeable to water.
Water is reabsorbed from the filtrate
Filtrate becomes concentrated
thin ascending LoH role
impermeable to water, but it is passively permeable to ions
moderately permeable to urea**
NaCl reabsorption
filtrate less concentrated
thick ascending LoH role
actively reabsorbs Na+ and Cl− ions from the filtrate via the Na/K/2Cl cotransporter - NKCC2).
dilutes the filtrate and concentrates the medullary interstitium
basis of the countercurrent mechanism
DCT
80 percent of filtered water has been recovered by the end of the LoH.
36 L of fitrate enters the DCT
another 10–15% is recovered here
Early distal tubule - impermeable to water
In the early DCT, Na+ enters via apical Na+ -Cl− cotransporters (NCC) - cotransported
Cl− leaves passively via basolateral channels.
Early DCT is also important for active transcellular Ca2+ transport - Regulated by parathyroid hormone
The late distal tubule has water channels and its permeability is regulated by antidiuretic hormone (ADH).
ADH also regulates urea absorption in the collecting duct
Combined effects of aldosterone and ADH on ENaC expression, urea transport and aquaporin expression fine tunes the ability of the kidneys to produce concentrated or dilute urine depending on the body’s needs.
• Low water intake = high water reabsorption
High water intake = low/no water reabsorption

2 types of cells DCT and CD
Principal cells – involved in NaCl transport
aldosterone dependant
Intercalated cells – participate in acid:base balance
help to get rid of acid generated by dietary intake (ie., fixed acid) that cannot be eliminated by the lungs
what controls sodium reabsorption in DCT and CD
aldosterone
what controls water reabsorption DCT and CD
ADH
countercurrent multiplier system
in LoH
nephrons with longer loops have greatest ability to concentrate interstitium
role of LoH; Generate and maintain the hypertonic interstitium
The term countercurrent comes from the fact that fluid is moving in opposite directions in the two limbs of the loop
3 characteristics
Countercurrent flow
Filtrate flowing down in descending limb and up in ascending limb
Descending limb permeable to water
Ascending limb impermeable to water
countercurrent multiplier; anatomical arrangement of LoH that concentrates solute in medulla
VERTICAL OSMOTIC GRADIENT
Enables water movement by osmosis down the concentration gradient and the Production of highly concentrated low volume urine
urea general info
• Urea is a small organic molecule comprising two amide groups joined by a carbonyl group
• It is formed in the liver
• Excreted in the urine to get rid of unwanted aa and nitrogen waste
• Normal plasma concentrations of 2.5–6.0 mmol/L.
when urea enters loop of Henle and gets recycled → helps to concentrate the surrounding intersitium of the inner medullary region of kidney
Many segments of the nephron are poorly permeable to urea
*as water is reabsorbed, urea is left behind; concentration in tubule rises
Some of the urea diffuses into the thin ascending limb of the loop of Henle and recycles
Urea recycling allows a high [urea] to be built up in the medulla
Urea can contribute 50% of the osmotic pressure of the medullary fluids in a maximally concentrating human kidney
![<p><strong>• Urea is a small organic molecule comprising two amide groups joined by a carbonyl group</strong></p><p><strong> • It is formed in the liver </strong></p><p><strong>• Excreted in the urine to get rid of unwanted aa and nitrogen waste </strong></p><p>• Normal plasma concentrations of 2.5–6.0 mmol/L.</p><p></p><p>when urea enters loop of Henle and gets recycled → helps to concentrate the surrounding intersitium of the inner medullary region of kidney</p><p></p><p><strong>Many segments of the nephron are poorly permeable to urea </strong></p><p>*as water is reabsorbed, urea is left behind; concentration in tubule rises</p><ul><li><p>Some of the urea diffuses into the thin ascending limb of the loop of Henle and recycles </p></li><li><p>Urea recycling allows a high [urea] to be built up in the medulla</p></li><li><p>Urea can contribute 50% of the osmotic pressure of the medullary fluids in a maximally concentrating human kidney</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9f57956a-6bf4-4418-afdf-4dccaf008f66.png)
nephritic syndrome presentation
proteinuria
micro heamaturia
high creatinine serum
due to glomerular inflammation
can also have HPT, low urinary output, oedema…
causes nephritic syndrome
due to glomerular inflammation
IMMUNE
systemic
infectious
diseases that classify as nephritic syndrome
rapidly progressing
ANCA
anti GBM
IgA
post strep
membranoproliferative glomerulonephritis
rapidly progressing disease
nephritic syndrome
can lead to renal failure
causes; ANCA, lupus, anti GBM…
macrophages bowman’s capsule
ANCA disease
nephritic syndrome
anti PR3/ MPO
ocular/ ENT symptoms
small vessels → lung/ lung hemmorhage
normal C3/C4
anti GBM
nephritic syndrome
small vessels→ lung
fatigue
antibody type 4 collagen
IgA disease
nephritic syndrome
most common
post inf/ strenous exercise
3 steps
IgA
C3→ normal levels
mesengial
henoch schloein; rash, scrotal pain, joints…
post strep disease
nephritic syndrome
2-4 weeks post group A beta strep
strep antigen
what nephritic syndrome can affect small vessels/ lung
ANCA
anti GBM
what nephritic syndrome can arise post infection
post strep
IgA
membranoproliferative glomerulonephritis
not a disease→ pattern of nephritic syndrome
tramline double membrane
mesengial proliferation
low C3/ C4
can also be nephrotic syndrome
C2/ C3
management nephritic syndrome
BP control
volume status control/ fluid restriction
immunosuppression
nephrotic syndrome presentation
occurs after podocyte damage
rise serum albumin
heavy proteinuria
oedema
jaundice
joint pain
rash
infection++
hypovolemia
causes nephrotic syndrome
primary
minimal change
membranous nephropathy
membranoproliferative nephropathy
focal segmental sclerosis
secondary
DM
lupus
hepatitis
amyloidosis
…
minimal chnage disease
nephrotic syndrome
erasure of cytokine
podocytes fusion
membranous nephropathy
nephrotic syndrome
anti PLA2R antibody vs antigen
IgG and C3 deposits
silver deposits
focal segmental sclerosis
nephrotic syndrome
IgM
black segments biopsy
investigations diagnosis nephrotic syndrome
24h proteinuria
protein; creatinine ratio
albumin; creatinine ratio
biopsy/ PLA2R atb
investigating cause nephrotic syndrome
FBC
viral serology
autoantibody panel
complement levels…
managements nephrotic syndrome
low fluids
low proteinuria/ ACEi
immunosupp? depend on cause
common complications nephrotic syndrome
hypercoagulability
infection risk
vit D deficiency
anemia
protein def
CKD
UTI pathogenesis
• Bladder normally sterile
• Anterior urethra colonised with skin or bowel flora
• UTI increases with age & is more common in women than men
• Children with UTI must be followed up, as renal failure & hypertension may ensue
determinants of infection utis
Inoculum size, how many bacteria
Virulence
host defence system
Complete bladder emptying (no culture medium)
Increase fluid intake & voiding frequency
Vesico-ureteral valve
Length of urethra (male>female)
Vaginal flora
decreased by oral contraceptives
urinary tract abnormalities
stones
reflux
irregular anatomy
UTI; men vs women
age vs uti
• Prostatic enlargement/hypertrophy
• Loss of bactericidal activity of prostatic secretions
• Faecal incontinence
• Pelvic floor muscle weakness, prolapse of the uterus leading to incomplete emptying of the bladder
host defence vs uti
• Regular flow of urine
• Mucosal defense mechanisms
• pH
• Integrity of sphincter
routes of infection UTI
Ascending
commonest by far
colonisation of ano-genital region with migration of enteric bacteria (Enterobacterales, enterococci) to bladder +/- renal pelvis
Haematogenous
Kidneys receive about 33% of cardiac output; bloodstream infection (BSI) may seed in the kidneys
responsible for <10% of infections, BUT spread from urine to blood far more common
Direct
fistula, e.g. vesico-colic
acute uti symptoms - localized
• Suprapubic pain (cystitis – inflamed bladder)
• Flank pain (pyelonephritis – inflamed kidney)
• Dysuria = pain when passing urine
• Frequency* = passing urine every 1-2 hours
• Urgency* = The urge to pass urine, must pass urine NOW!
• Nocturia* = passing of urine during night which is out of usual habit
acute uti symptoms - systemic
• Fever
• Rigors
• Acute confusional state/ delerium in elderly
• Nausea, anorexia
• Obstructive uropathy may contribute to acute kidney injury and associated symptoms
UTI CAUSATIVE PATHOGENS
gram neg bacilli
E. coli
Klebsiella pneumoniae
Proteus mirabilis
Pseudomonas aeruginosa
gram pos cocci
staph
group B strep
Enterococcus faecalis
E coli vs UTI
the most common pathogen causing UTI
gram neg bacilli
staph vs uti
coagulase-negative staphylococcus – can be part of the normal flora
Another common cause of UTI in the community
Tends to affect young women
gram pos cocci
enteroccocci vs uti
An opportunistic pathogen and not particularly virulent
Complicated infection in critically ill or immunocompromised patients
gram pos cocci
PSEUDOMONAS AERUGINOSA vs uti
gram neg bacilli
Opportunistic pathogen
Not a common cause of UTI
Complicated infection in critically ill or immunocompromised patients or structural urinary tract abnormalities
Characteristic sweet odour
HCA uti
Pseudomonas aeruginosa
Enterococcus faecalis/ faecium
Predisposing factors:
Presence of urinary catheters
Manipulation of the urinary tract – TRUS-guided prostate biopsy, stone fragmentation, stenting, urinary diversion – nephrostomy, ileal conduit
Urinary stasis
Dehydration
Debility due to underlying disease
how to prevent HCA uti
• Standard precautions including hand hygiene – every patient, every time
• Use antibiotics appropriately and follow the guidelines – reduce the risk of antimicrobial resistance
• Mind the devices:
only use catheters when necessary
remove when no longer necessary
Insert the catheter using an aseptic technique
diagnosing UTI
CONFIRMATION REQUIRES BOTH:
1. PRESENCE OF CLINICAL SYMPTOMS
2. SUPPORTING EVIDENCE FOR UTI
tests
MSU/ CSU
dipstick - not a confirmation on its own
culture/ micro/ susceptibility
blood - rule out BSI
Asymptomatic bacteriuria
bacteria in urine without symptoms of UTI
more common with aging/ catheter
not a diagnosis/ should not be treated unless
Pregnancy
If untreated, 20-30% will develop acute pyelonephritis (AP)
Manipulation of the urinary tract
URINE DIPSTICK/URINALYSIS
look at colour of sample/ cloudy
Protein
Blood
Glucose
Ketones
Leucocytes
Nitrites
DIPSTICK IS A GOOD TEST TO RULE OUT UTI:
NEGATIVE for nitrites and leucocytes = UTI very unlikely
POSITIVE for nitrites and leucocytes = Careful interpretation needed
microscopy findings uti
• White blood cells (WBC) or pus cells: normally <10
• Red blood cells (RBC): calculi or glomerulonephritis, tumours or cystitis
• Epithelial cells: presence may indicate specimen contamination
• Bacteria: visible bacteria on microscopy = bacteriuria
• Casts
INTERPRETATION OF THE COLONY COUNT uti
• >10^5 /mL – supports UTI diagnosis, provided patient has symptoms of UTI
• 10^4 /mL – interpret with caution – review microscopy, is the patient symptomatic, was the patient on antimicrobials before the specimen was taken?
• 10^3 /mL - probable contamination
• Mixed growth – likely contamination, send repeat specimen only if clinically indicated