Inorganic chemistry year 3
1/194
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
195 Terms
Labile
Low charge density (small charge, big radii)
TM gains stability (+ve change in CFSE) going to 5- or 7- coord, lower E barrier
d0, d1, d2, d4 HS (c4v), d5 HS, d6 HS, d7, d9 HS (c4v)
Inert
High charge density (high charge, small radii)
TM loses stability (-ve change in CFSE) upon forming coord - higher E barrier
d3, d4 HS (d5h), d4 LS, d5 LS, d6 LS, d8, d9 (d5h)
A mechanism
Limiting rates not observed
D mechanism
Ligands react faster than rate for solvent exchange
Small variation in rates
Ia mechanism
Rate dependence on nature of Y (bond formation dominates)
Rates can vary by big amounts
Id
Independent of Y
Limiting rate slower than solvent exchange
Thermodynamic activation parameters for A mechanism
negative -delta S activation
negative - delta V activation
Thermodynamic activation parameters of D mechanism
positive + delta S activation
positive + delta V activation
Reactions of square planar complexes
Solvent interactions = reaction accelerated by Nu, slowed by sterically bulky co-ligands
2 consecutive A exchanges
Trans influence
Sigma donors
More effectively donates e density to M
Exerts repulsion on LG, weakening M-X
Trans effect
Strong pi acceptor ligands
Pull e density away from M, stabilising 5 coord intermediate
If you want a trans isomer you have to start with adding…
The less labile ligand
Labilising series
Pi acceptor ligands prefer M in…
Low Oxn states
Sigma bonding ligands prefer…
M in high oxn state
Ligands that donate 1e-
H, Cl, Br, CN, bent NO, Me, Ph, COMe
Ligands that donate 2e-
, CO, PR3, P(OR)3, CNR, N2, O2, CR2, C2H4, NH3, CN
-charge ligands (wouldn’t exist by themselves)
Me, Et, Cl, Br, Cl, RO, RS, HO, R2P, R2N, Ph, C5H5, C(O)R
Neutral ligands
I2, R2O, H2O, R2S, PR3, NH3, NR3, CO2, CO, N2, C2H4, C6H6
pi acceptors
EWGs increase pi acidity
phosphines can be affected by…
steric factors - cone angle, bulkier R groups on P, bigger cone angle
electronic factors - e.g. if R = EWG, less backdonation of e density from M to pi* of CO - CO bond stronger
heterogeneous vs homogeneous catalysis
TM with carbonyls
TM with phosphines
ASSEMBLY - transmetallation
no change in anything
TM-X + M-R e.g.: Cl + PhLi → Ph + LiCl
ASSEMBLY - substitution
no change in anything
one 2e donor replaced by another
ASSEMBLY - addition
2e donor added
VE increase by 2
CN increase by 1
ASSEMBLY - oxidative addition
adddition of X-Y (add separately)
2e addition
VE increase by 2
OS increases by 2
CN changes by 2
Me-I (easiest to break) > H-H > Me-Me > Me - H
MODIFICATION - 1,1 ME
R inserted into CO ligands
MUST BE CIS
VE decrease by 2
CN decreases by 1
MODIFICATION - 1,2 ME
R inserted into alkene
MUST BE CIS
H inserts at more sub end of double bond, reducing steric hindrance on M
VE decreases by 2
CN decreases by 1
MODIFICATION - nucleophilic attack
alkene bound to TM (e density pulled away by TM) can react with nu
REQ TM w high oxn state, formal +charge, other EWGs coord
trans attack - attack at more subbed end of alkene
attack at M (req e deficient M that can add another ligand) - cis addition at less subbed end of alkene
EXPULSION - 1,1 RE
elimination of X-Y
MUST BE CIS
VE decreases by 2
OS decreases by 2
CN decreases by 2
EXPULSION - B-hydride elimination
M-C-C-H → alkene ligand
can then undergo 1,1 RE
EXPULSION - a-hydride elimination
M-C-H → carbene M=C-R
Vibronic coupling
relaxation of laporte rule
g-g transition allowed
e.g.: asymmetric vibration destroys inversion centre
Why are d-d transitions allowed in Td complexes?
dp mixing
Spin orbit coupling
relaxation of spin selection rule
good for 2nd and 3rd row TMs (heavy atom effect)
Pi donors have _ Δo
small
weak field ligands
high spin
Pi acceptors have _ Δo
high
strong field ligands
low spin
1st row TMs have _ Δo
low
high spin
Free ion terms *
θ = 360/no of axis
B - racah parameter
B(free ion) > B(complex)
e repulsions weaker in complexes
small B = signif delocalisation of e to ligands
softer ligands (pi acceptor)= smaller B
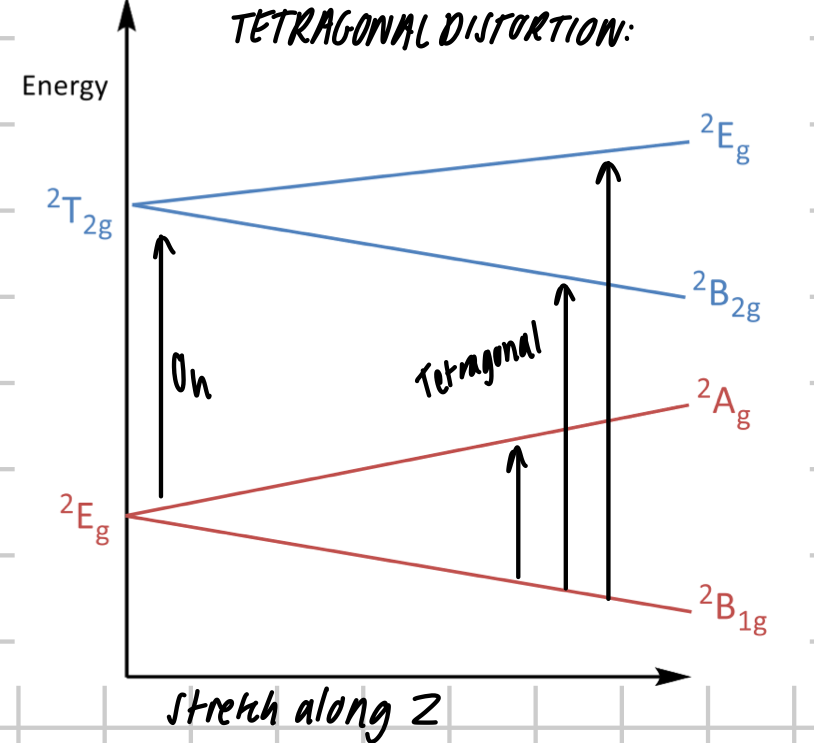
What happens when there’s a tetragonal distortion from Oh → D4h
symmetry change
additional transitions possible
LINE BROADENING → shoulder peak
LMCT (general)
more intense than d-d (Laporte allowed)
more likely when ligands have lone pairs - PI DONORS
OR M has high oxn state - RHS d-block, 2nd+3rd row TMs
LMCT Oh
E decreases across TM series (3d orbital E decreases w increase in Zeff)
E decreases down triad (d orbital-ligand overlap increases)
LMCT Td
E decreases as ligands are less electronegative (E of p orbitals increases)
E decreases as no of ligands decreases (less e repulsion - E of M orbitals decreased)
MLCT (general)
most likely when ligands have low pi* - PI ACCEPTORS
m is in low oxn state
E increases across TM series (d orbital E decreases as Zeff increases)
E increases down triad (d-orbital-ligand overlap decreases?)
Spin forbidden d-d transitions can give rise to sharp abs bands when…
transitions involve spin flip in orbitals
d3 and d5?
Spin crossover (LS→HS)
change in number of bands (d-d transitions) - d6 LS = multiple bands, d6 LS = one band?
increase in bond length (e occupy Eg antibonding)
increase in mag moment
ligands remain the same
usually only in 1st row TMs
d4 to d7 only - can be high or low spin
Spin-only magnetic moment
1st row only - no signif orbital contribution
u = 2(S(S+1))^1/2
why is ΔV activation easier to calculate than ΔS activation?
gradient rather than intercept (subject to larger error)
harder to obtain experimentally
Outer sphere electron transfer
e transfer occurs bw two molecules w/o bond breaking
Inner sphere electron transfer
single ligand links 2 M ions
bond breaking occurs
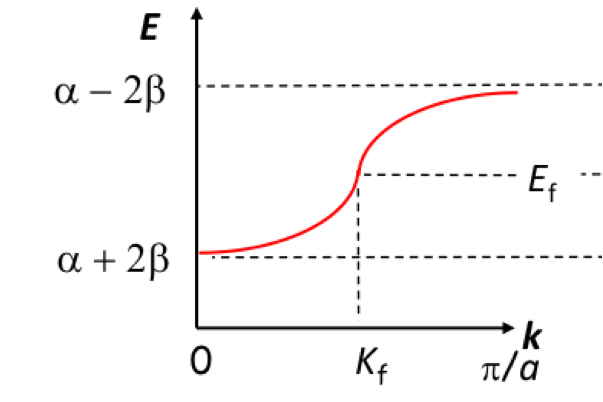
Fermi energy
E at which the probability of the levels being occupied is 0.5
Group 2 band structure
S band filled
In 3D, s and p overlap (top of s band higher E than bottom of p band)
Group 2 change w pressure
Down group, as P increases, conductivity decreases
Start of group, overlap good > sp gap small (avoided crossing)
Increased P, atoms closer together (more overlap) → bonding E lowered, antibonding E raised → band separation/gap increases → bands overlap less in 3D → conductivity decreases
Group 12 band structure w increased P
orbital overlap bad < s-p gap - no avoided crossing
under P, band gap narrows → bands overlap more in 3D → conductivity increases
Bloch function
periodic functions defining the wavefunctions for a 1-D chain of N atoms
value of the wavelengths must be the same on both ends of the chain
wavefunction must have periodicity that reflects the regular repeat spacing a bw atoms
wavefunction must be normalised
Sizes of bandgap…
Insulator > semiconductor > metal
Fermi level for metal
E up to which energy band is filled
Fermi level for a semiconductor
Midpoint energy in bandgap
Band theory
extension of MO theory
overlap of valence orbitals creates closely spaced E levels that forms bands
Jahn-Teller effect
Distortion when orbitals aren’t degenerate
e.g.: d3, d5 excused
Huckel theory (a and b)
a = E of relevant AO
B = interaction of AOs
important for describing (in)stability of bonding
Energy level diagram (band theory)

Band structure diagram (E vs k)
Density of states diagram (1D)
Band structure of group 1
Half filled s orbitals overlap → half filled band
Therefore metals
What is k (adrian)
WAVENUMBER - potential E levels bw all antibonding/bonding
all linear combos of AOs characterised by a value of k
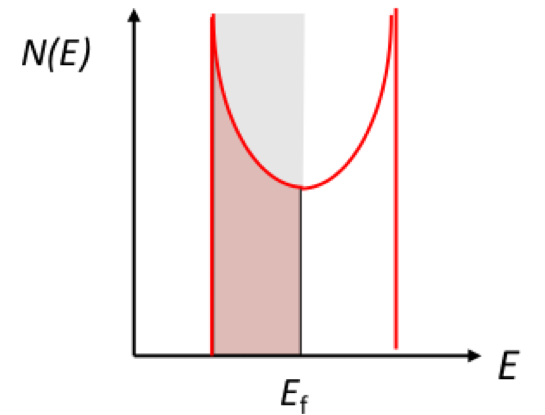
Why can only a small amount of e near Ef access higher E levels?
kBT (energy in environment) at 300K ~ 0.025eV - only small % can access higher E level
s and p band mixing scenarios for group 2 (1D)
band dispersion (broadness of band from overlap of orbitals) < s-p gap → separate s + p bands exist
band dispersion (broader bands) > s-p gap →1xsp bonding, 1xsp antibonding band w gap → avoided crossing
2D band structure
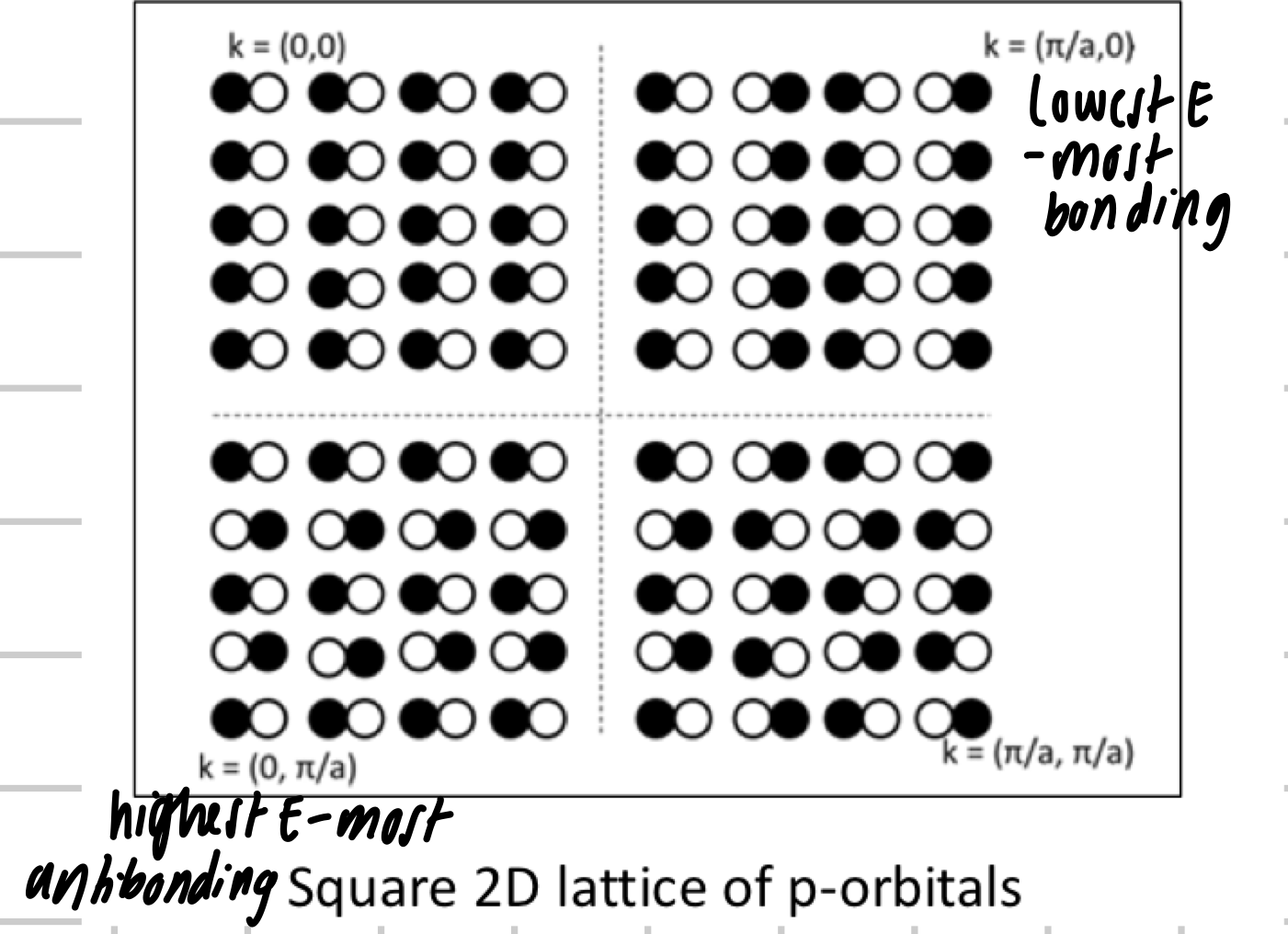
Why can metals absorb a wide spectrum of energies in the visible spectrum?
band widths E > E of visible light
Resistivity scattering (optical properties of metals)
electrical resistance caused by collisions in the metal lattice w imperfections in lattice (lattice imperfections or lattice vibrations, increase w T)
causes rapid re emission of light as e drop back to ground state
How can some TMs show ferromagnetism?
TMs w a d-band at the fermi level have a high density of states (many degenerate levels)
nearly full, narrow 3d band (d orbitals less diffuse more localised)
E favourable to have high number of e w parallel spins →
allows significant no e to move to unpaired states above fermi level
B (interaction integral) is _ for d (compared to s+p)
Much smaller
At 0K, group 4 allotropes w diamond structure are…
Insulators - no electrical conductivity
band gap at 0K - no thermal E to promote E to CB
Above 0K, for group 4 elements…
thermal excitation is possible
depends on thermal E available and band gap
Band gap of insulators
Band gap > 3-4eV
Band gap of semiconductors
0-3/4eV
Band gap of metals
~0
The bigger the band gap…
The lower the conductivity
Conductivity increases with T for…
Semiconductors
Insulators
Conductivity decreases with T for…
Superconductors
Metals
Extrinsic semi-conductor (n-type)
e.g. Si doped w P
extra e remains near P
donor level formed just below conduction band
e can be easily ionised into CB
*add image
Extrinsic semiconductors (p type)
e.g.: dope Si w B
each group 3 provides one less e to VB than Si
e promoted from VB to acceptor level just above VB (where conductivity occurs)
*add image
Down group 4…
down group, r increases (covalent bond strength decreases) → decreased orbital overlap → decreased band gap (sp3 bonding raised, sp3 antibonding lowered) → bands overlap more in 3D → better conductance
Upon increasing temp Ef…
Goes from an extrinsic region to intrinsic region
What are most insulators?
Ionic compounds (oxides, halides)
(filled valence bands)
What are most semiconductors?
covalent e.g.: sulphides
(filled valence bands)
Increased difference in electronegativities
increased charge transfer
increased E of band gap
more insulator character
decreased colour
Why do energies of donor/acceptor levels in extrinsic semiconductors not depend on dopant species?
e delocalised over many host atoms
overall lattice properties more important
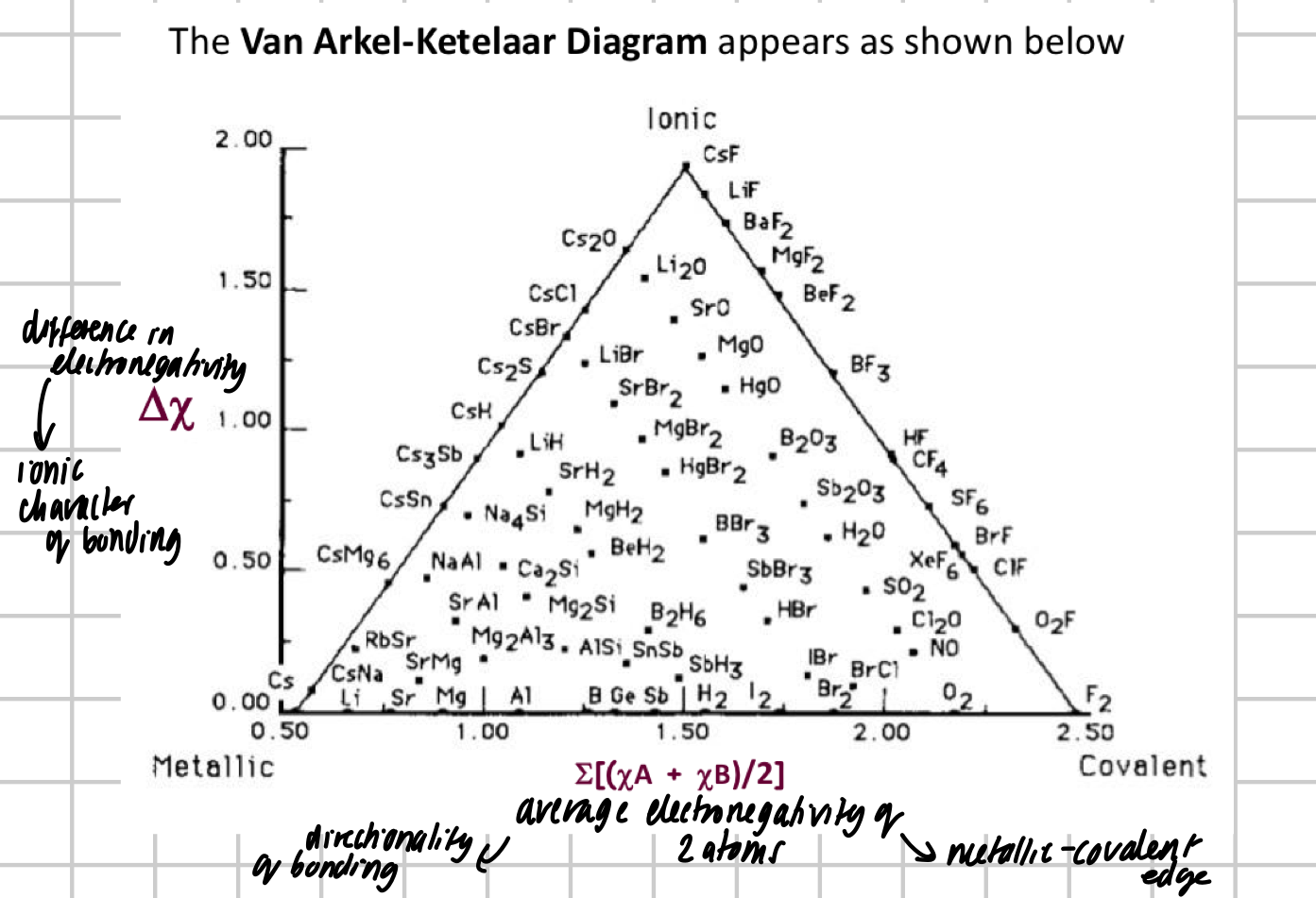
Van Arkel and Ketelaar diagrams
Low avg electronegativity = metallic
High avg electronegativity + big difference = ionic
High avg electronegativity + small difference = covalent
How does a p-n junction act as a diode?
allows current to flow in 1D
e move from n-type through junction
holes move from p-type through junction
if electric field reversed, current can’t flow, e and h move away from junction
LEDs vs PVs?
LEDs - voltage applied → e + h recombine at junction + emit light
PVs - absorb light → voltage to power external work
Change of Ef with T in semiconductors
B = (adrian)
B = uH
B - response of volume (magnetic induction - Tesla)
H - mag field strength (amps m-1)
u or uo = permeability of volume/free space (vacuum)
What happens to a diamagnet in a magnetic field?
decreased density of lines of force - repelled by mag field
Magnetisation is negative (-)
What happens to a paramagnet in a magnetic field?
increased density of lines of force - attracted by mag field
Magnetisation (M) is positive (+)
B = (when mag field applied)
B = uoH + uoM