GCSE Pearson Edexcel Separate Science Chemistry: Atoms
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds. For example, water.
Element
A pure substance made of only one kind of atom For example, a diamond is made of pure carbon!
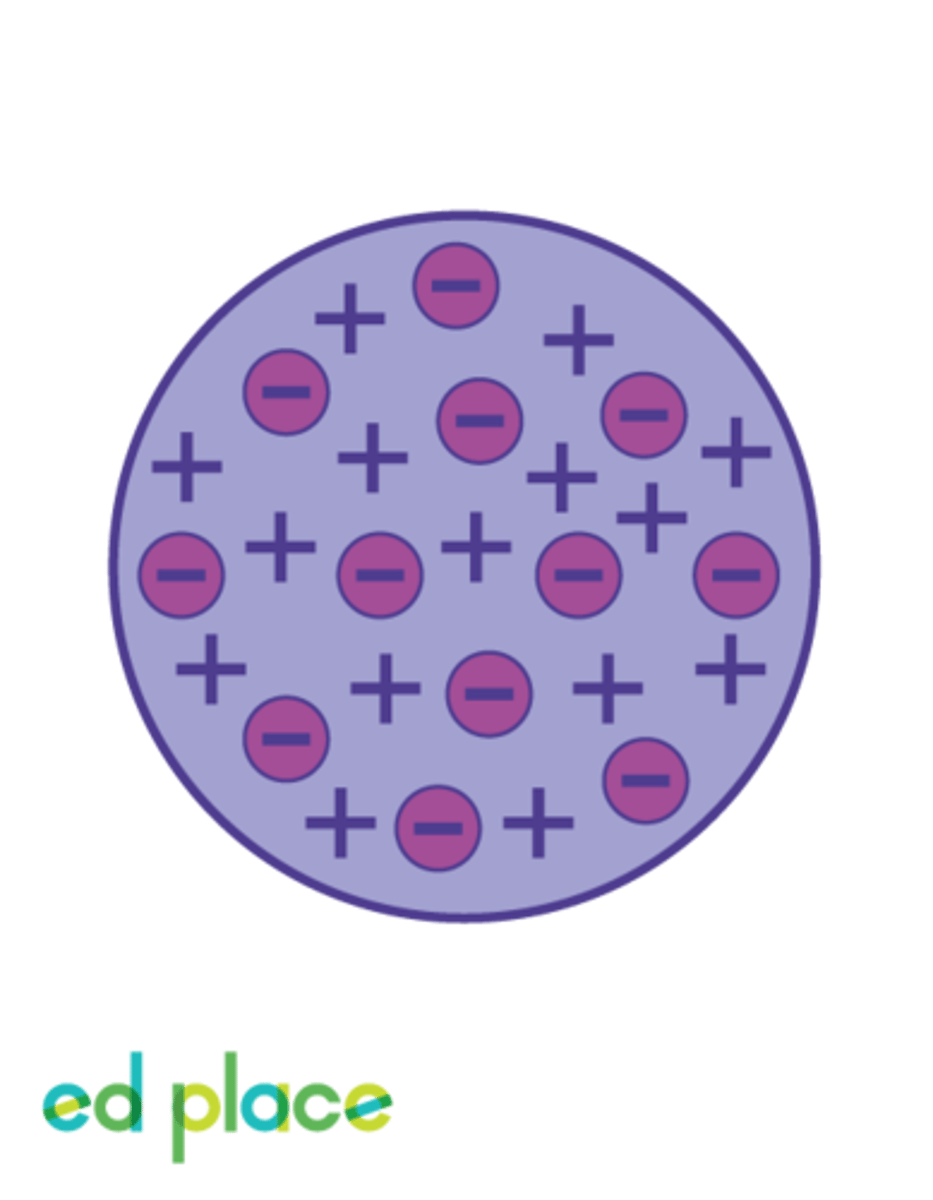
Plum Pudding Model (Thomson)
JJ Thomson's atomic model which depicts a positively charged space containing negatively charged electrons
Electrons
Negatively charged particles
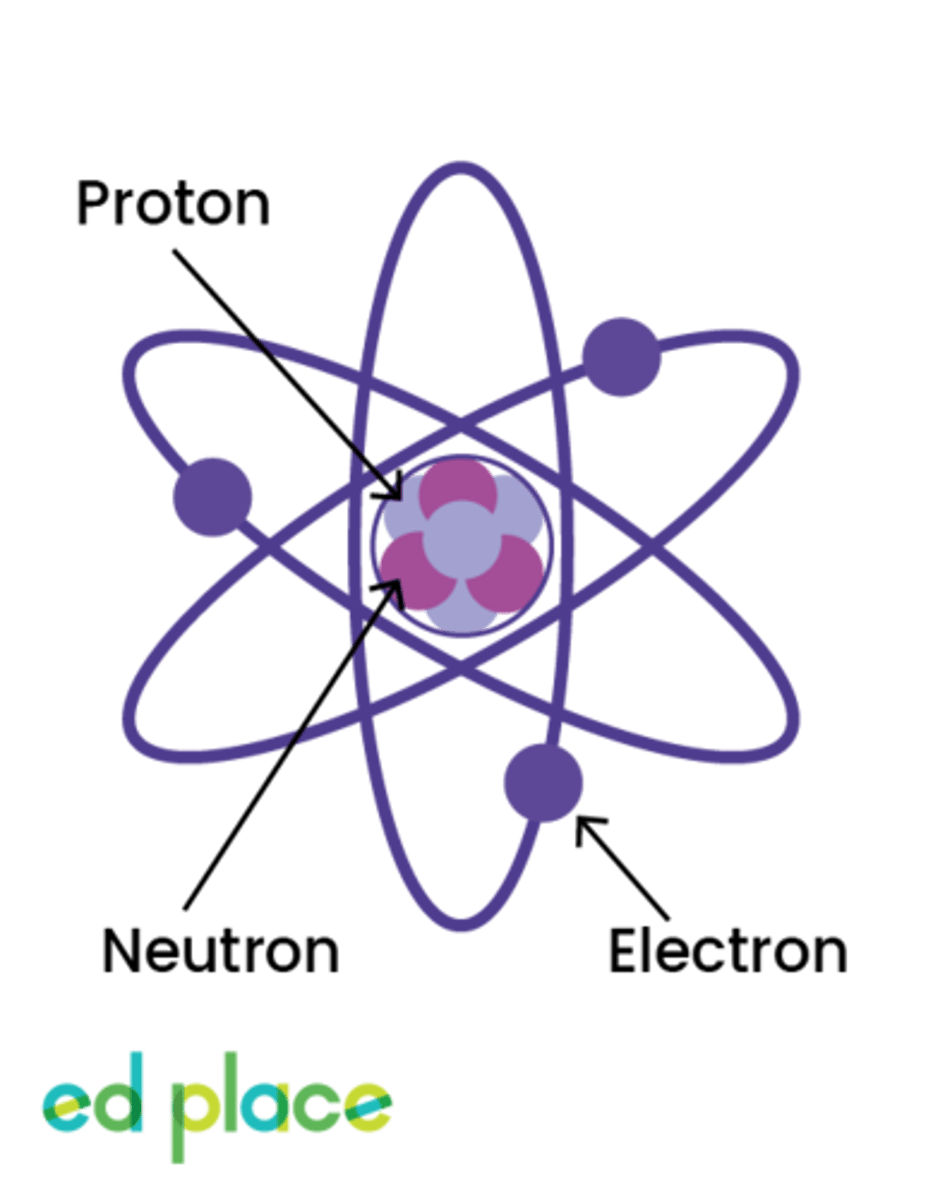
Proton
A particle that has a positive charge and is found in the nucleus of an atom
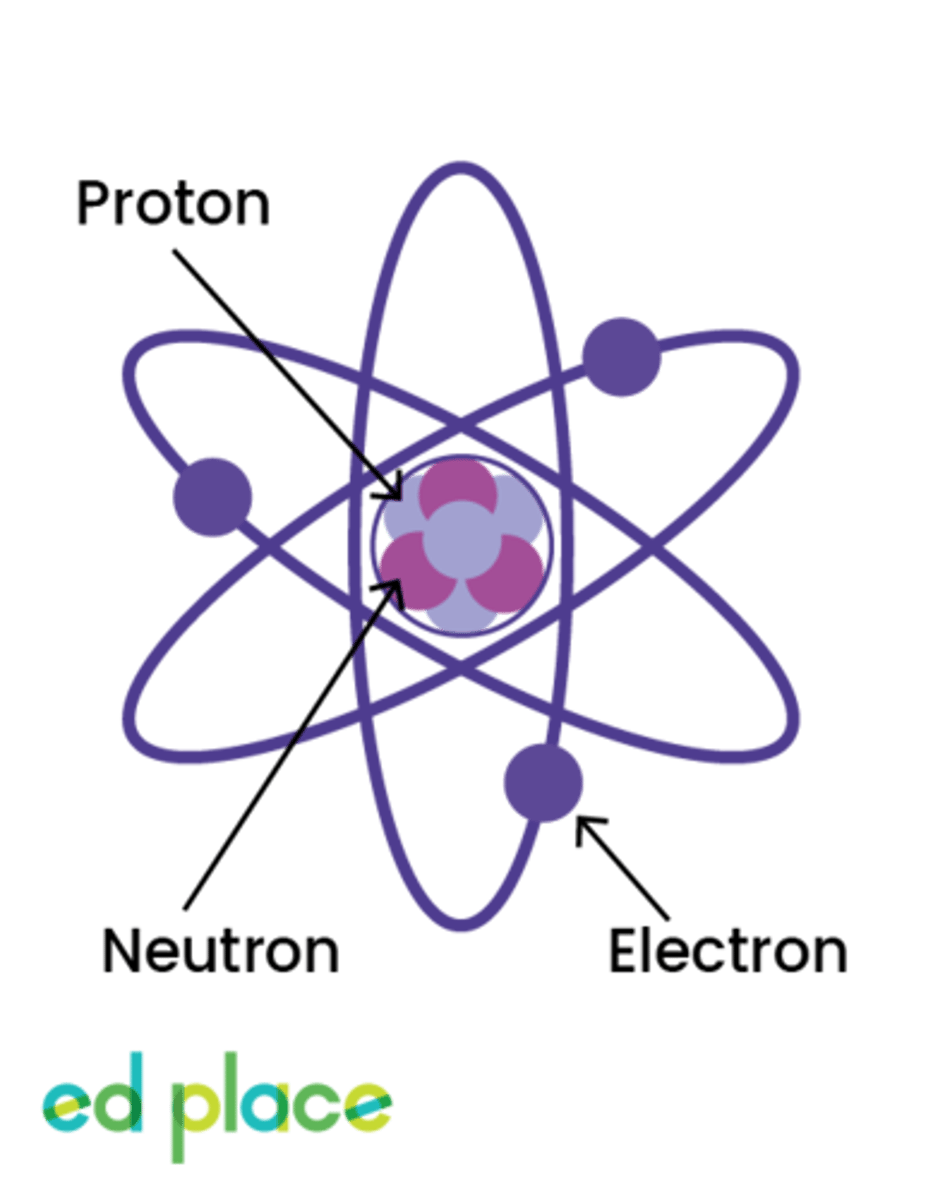
Sodium
Atomic Number: 11
Symbol: Na
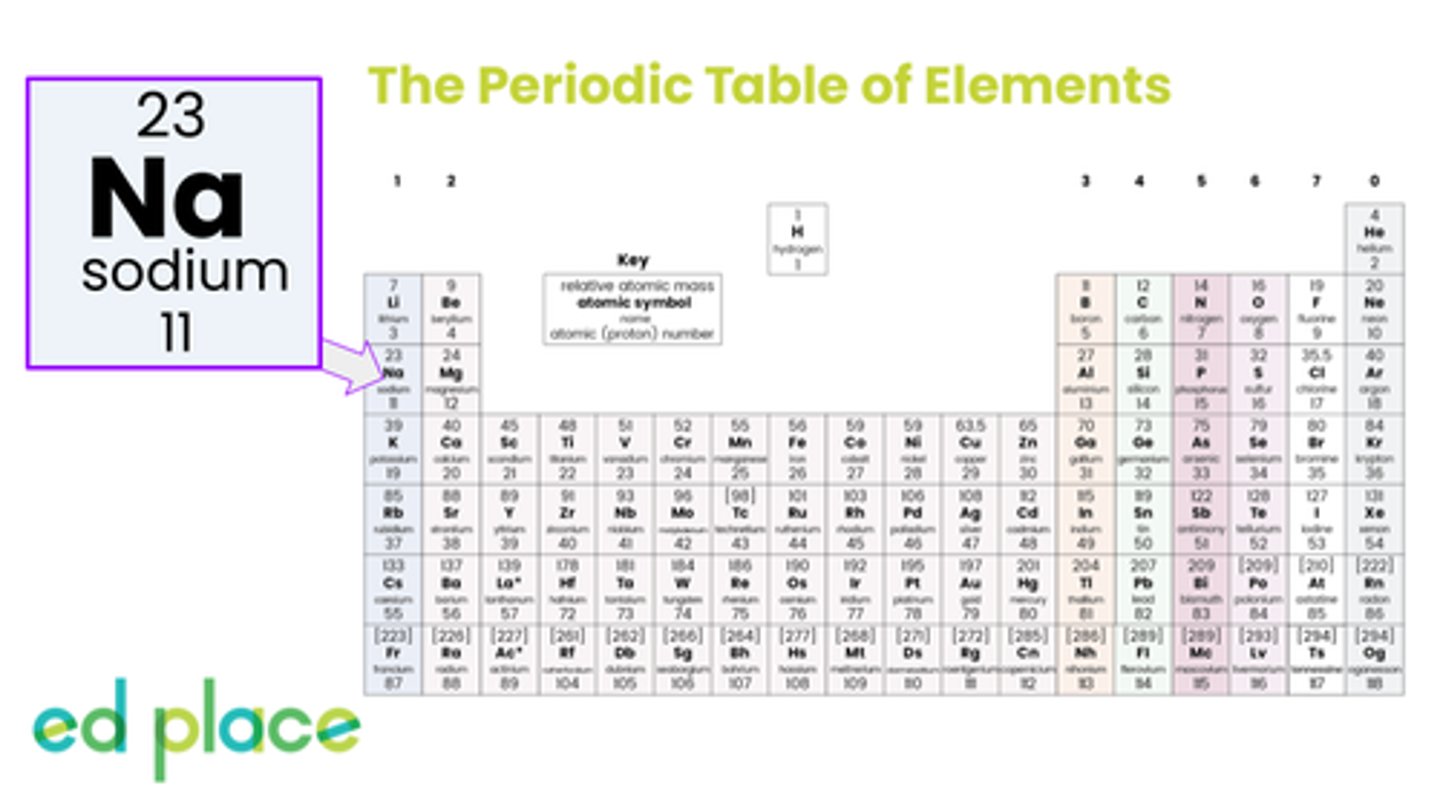
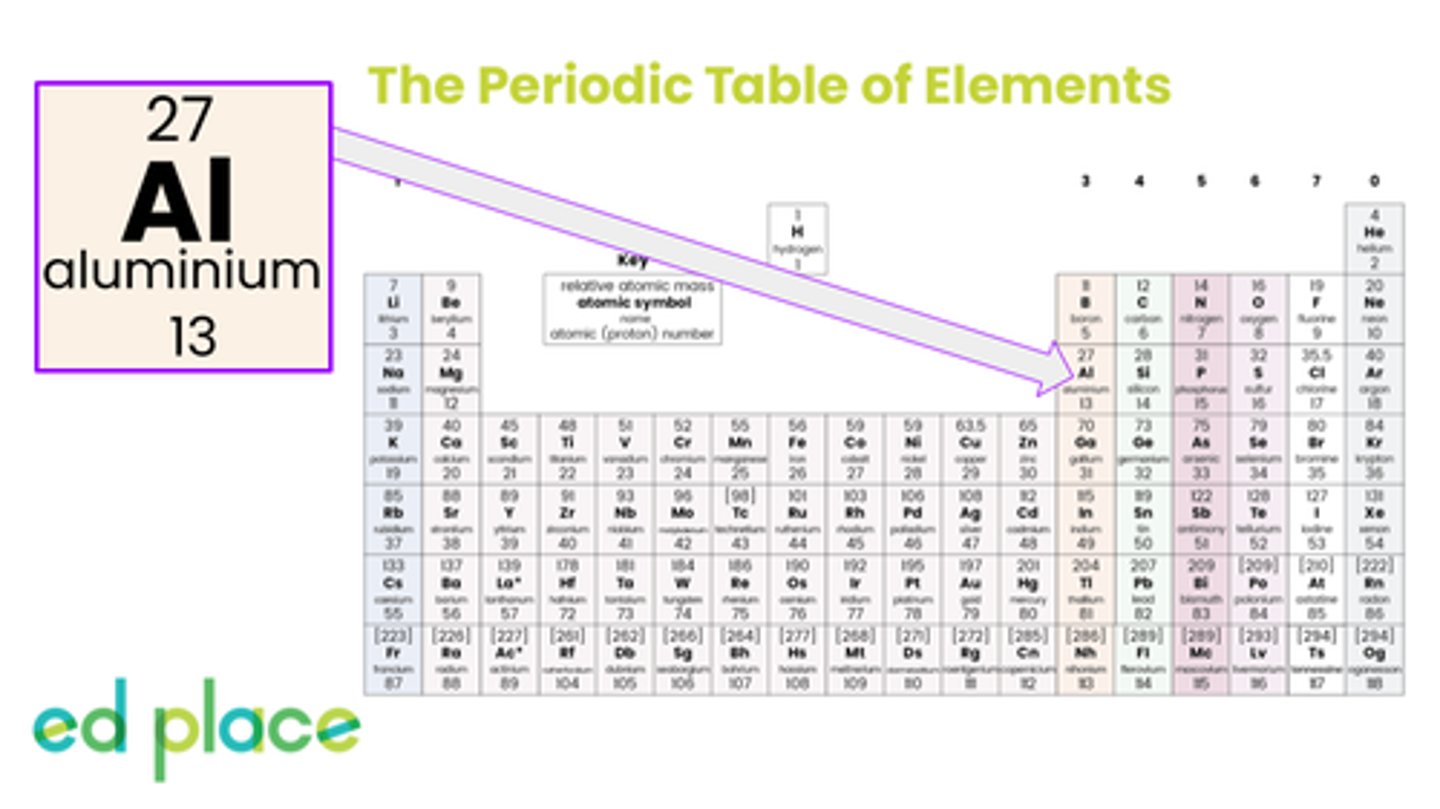
Aluminium
Atomic Number: 13
Symbol: Al
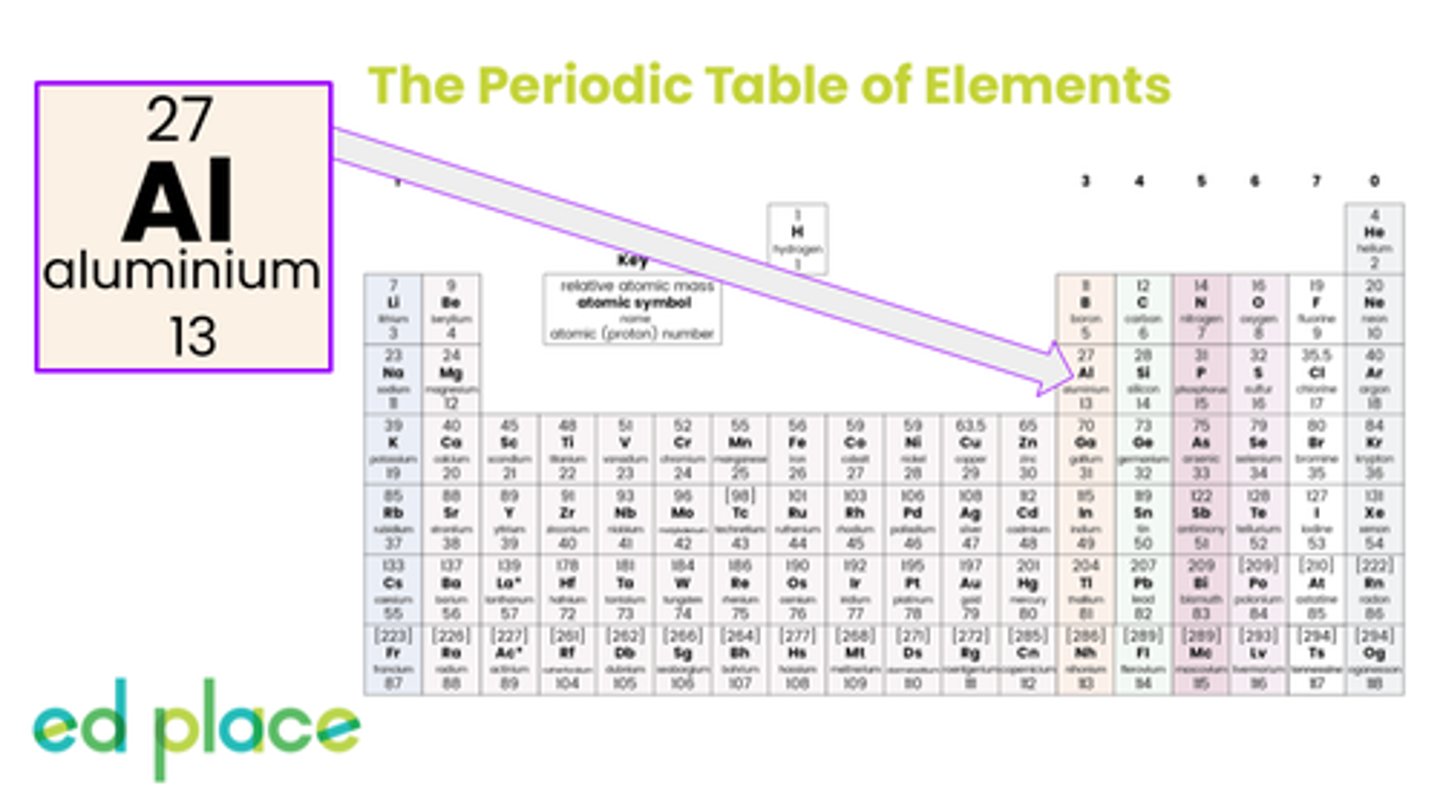
How many neutrons does an aluminium atom have?
14

How many electrons does an aluminium atom have?
13
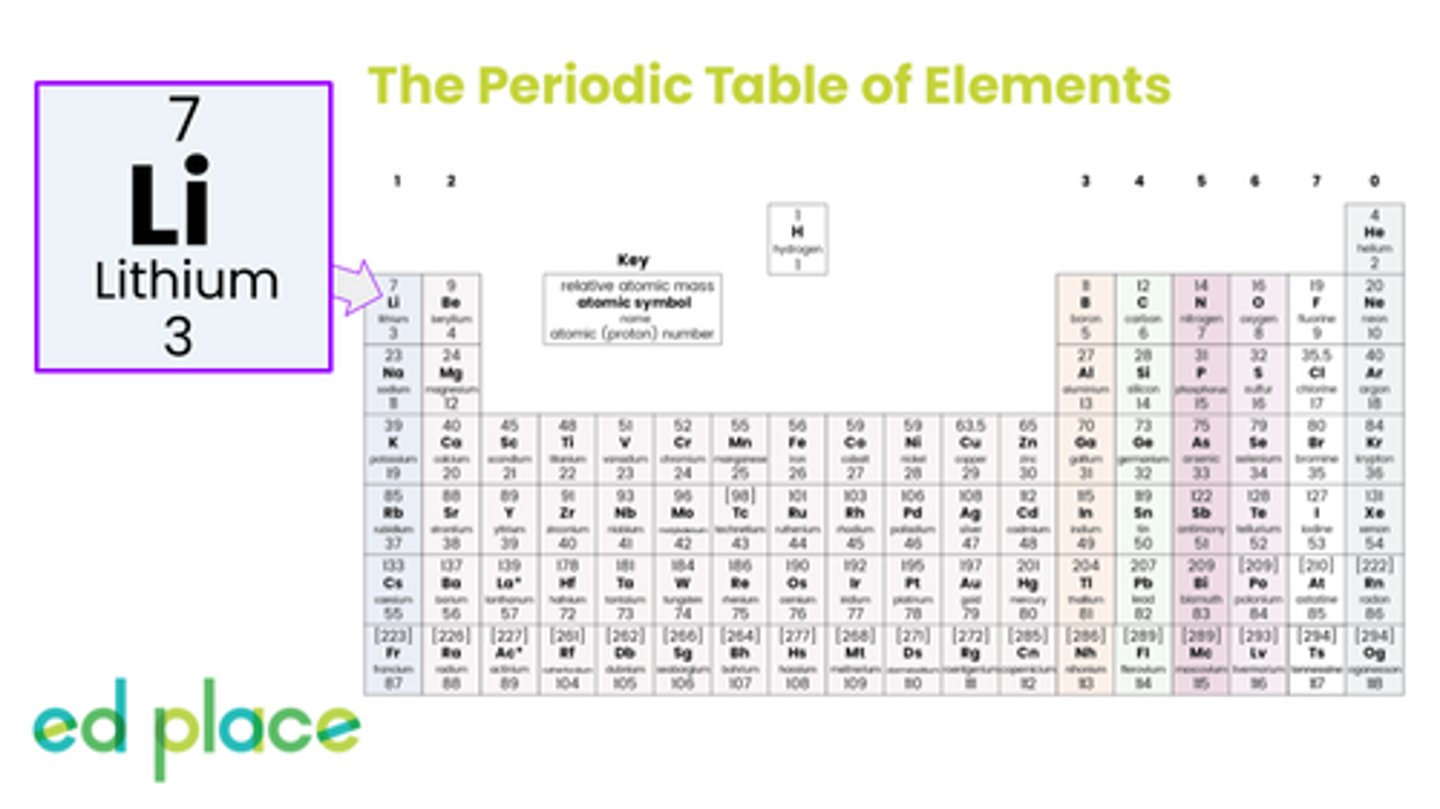
What is the mass number of lithium?
7
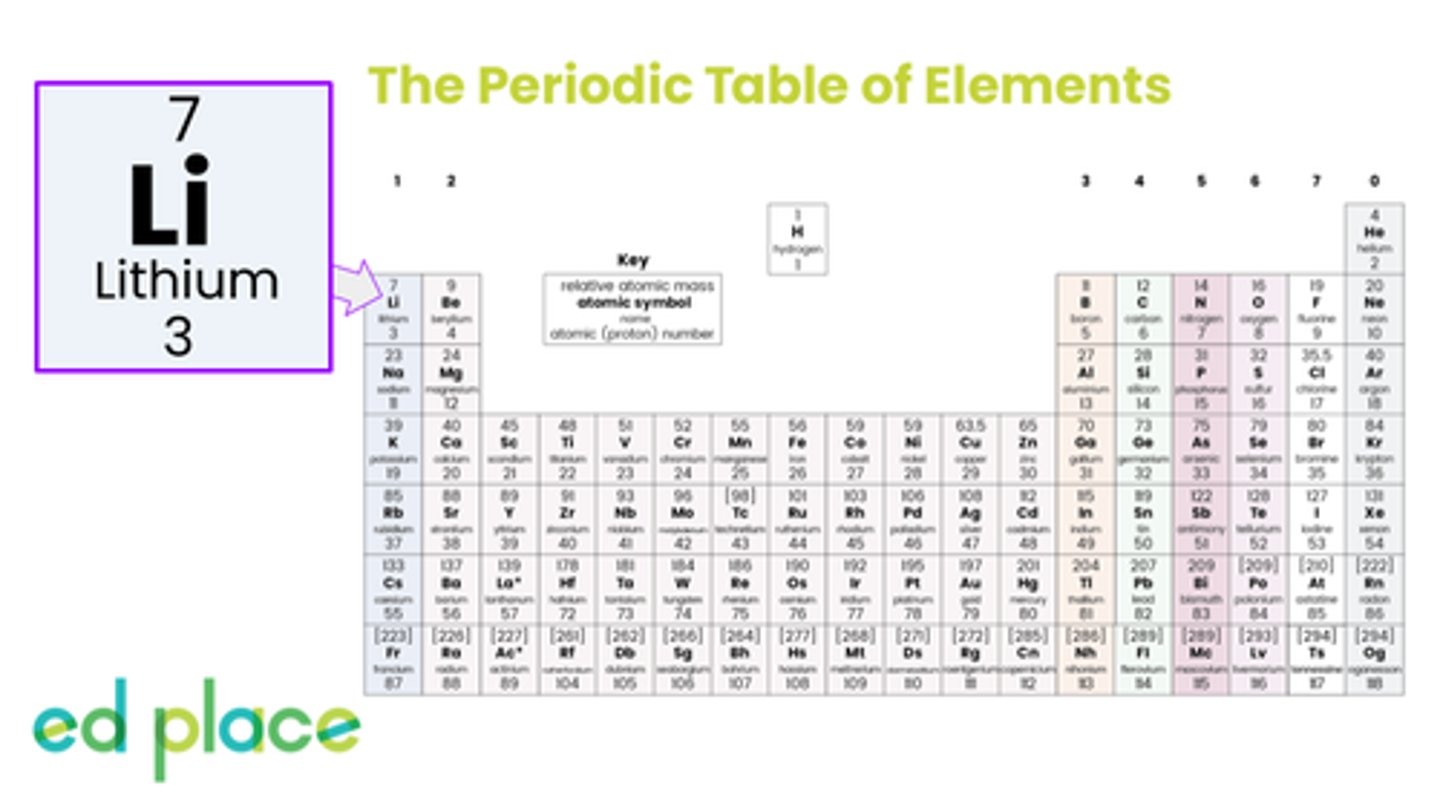
How many electrons does a lithium atom contain?
3
How many protons are there in an atom of potassium?
19
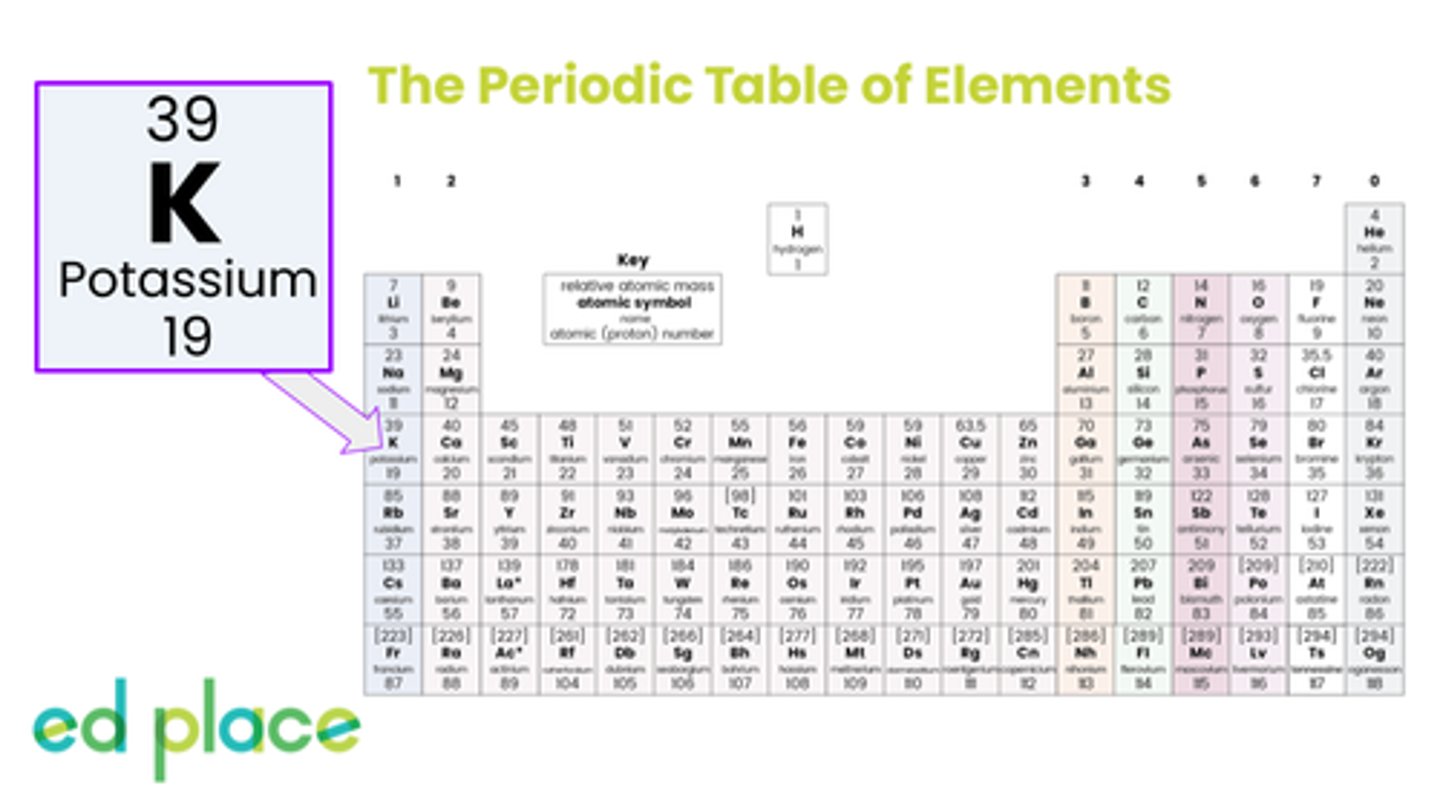
How many neutrons are there in an atom of potassium?
20

Can you draw a sodium atom?
Sodium
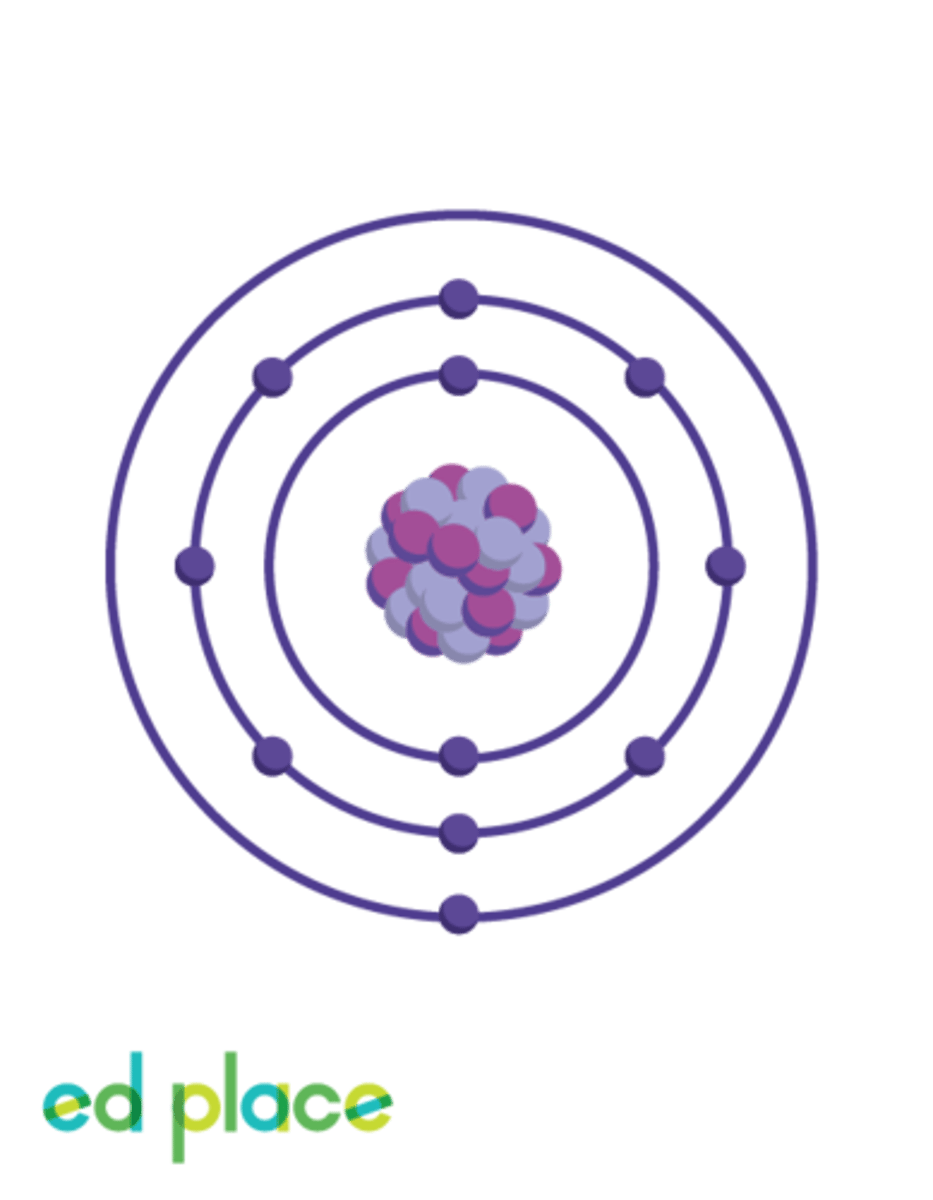
How many electrons does a sodium atom have?
11
What is the electron structure of a sodium atom?
2.8.1
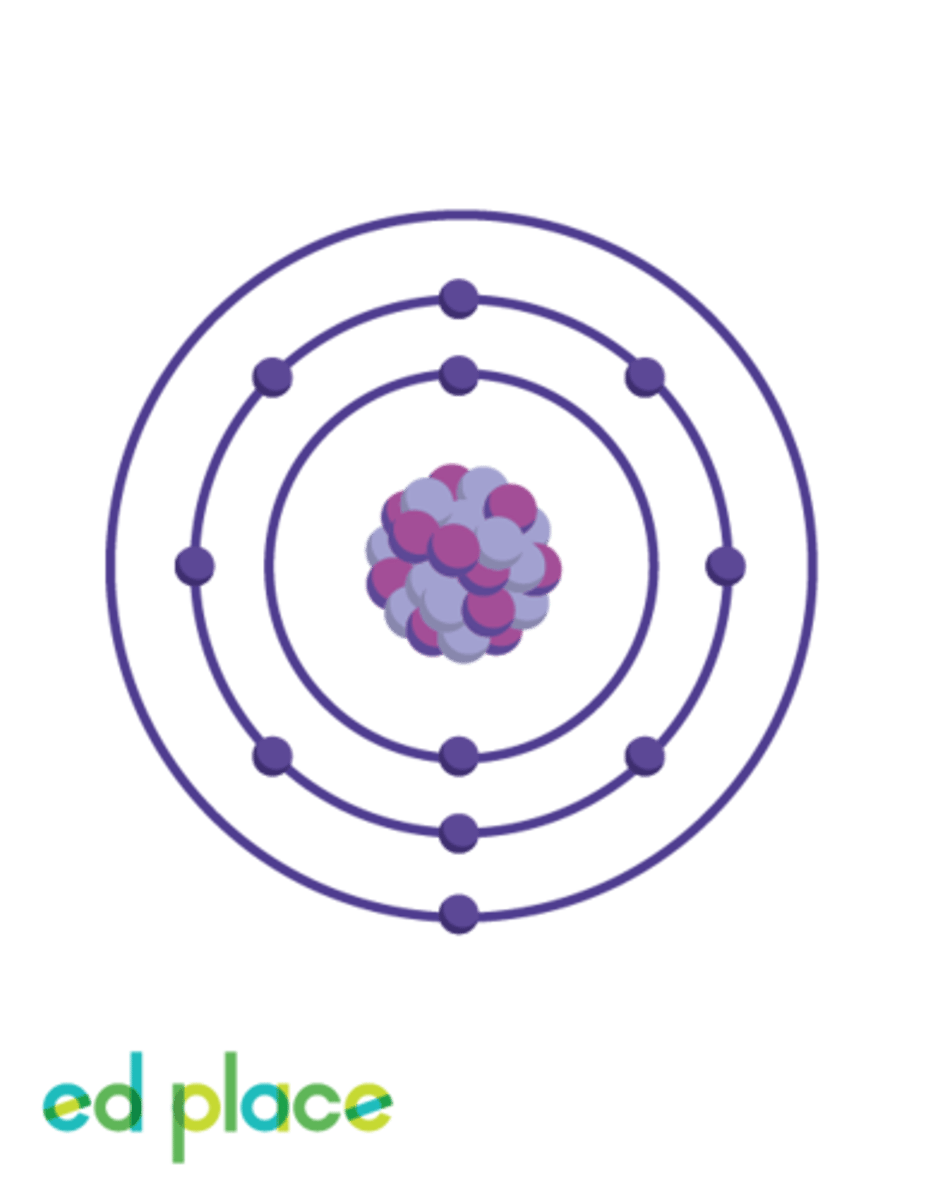
How many electrons are there in an aluminium atom?
13
What is the electron structure of aluminium?
2.8.3
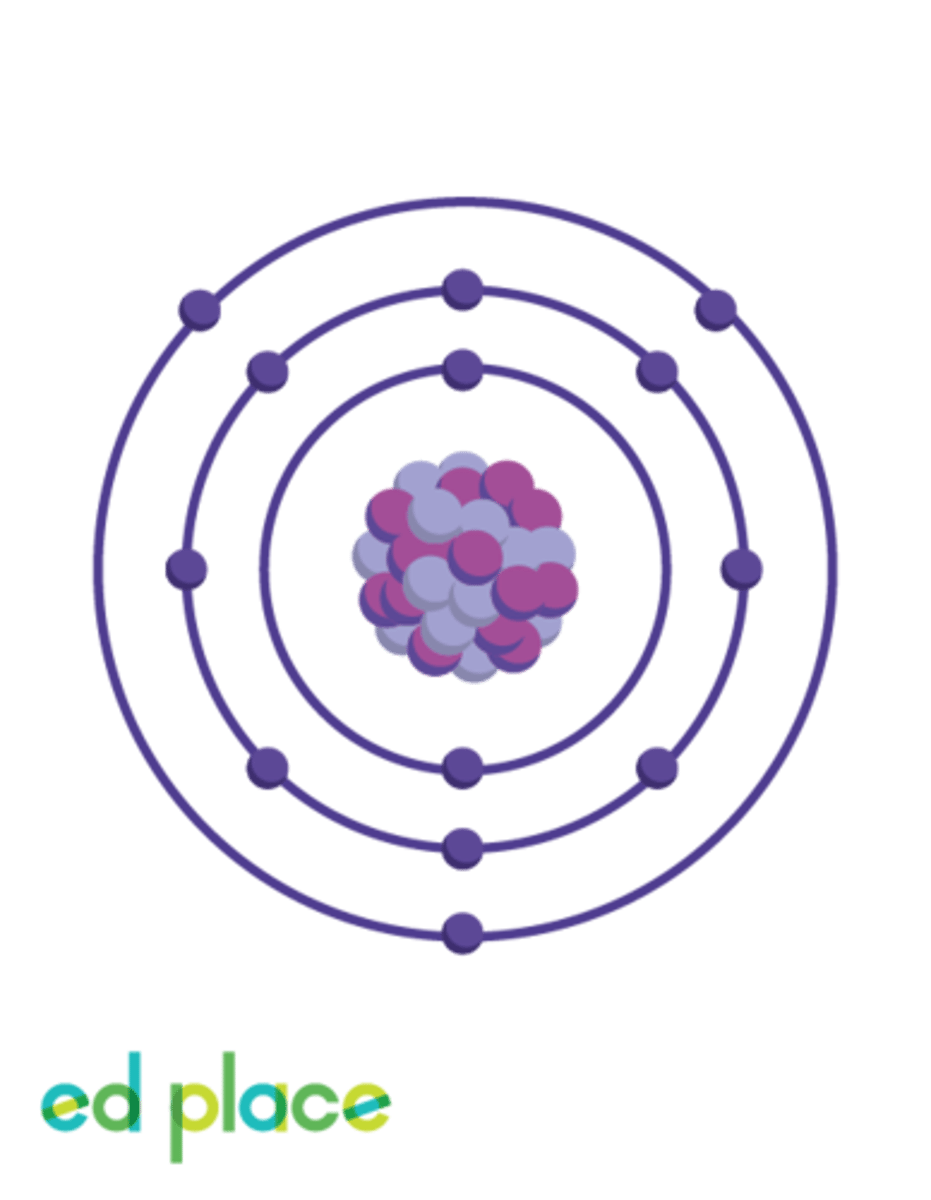
What group can aluminium be found in?
Aluminium is a group 3 element
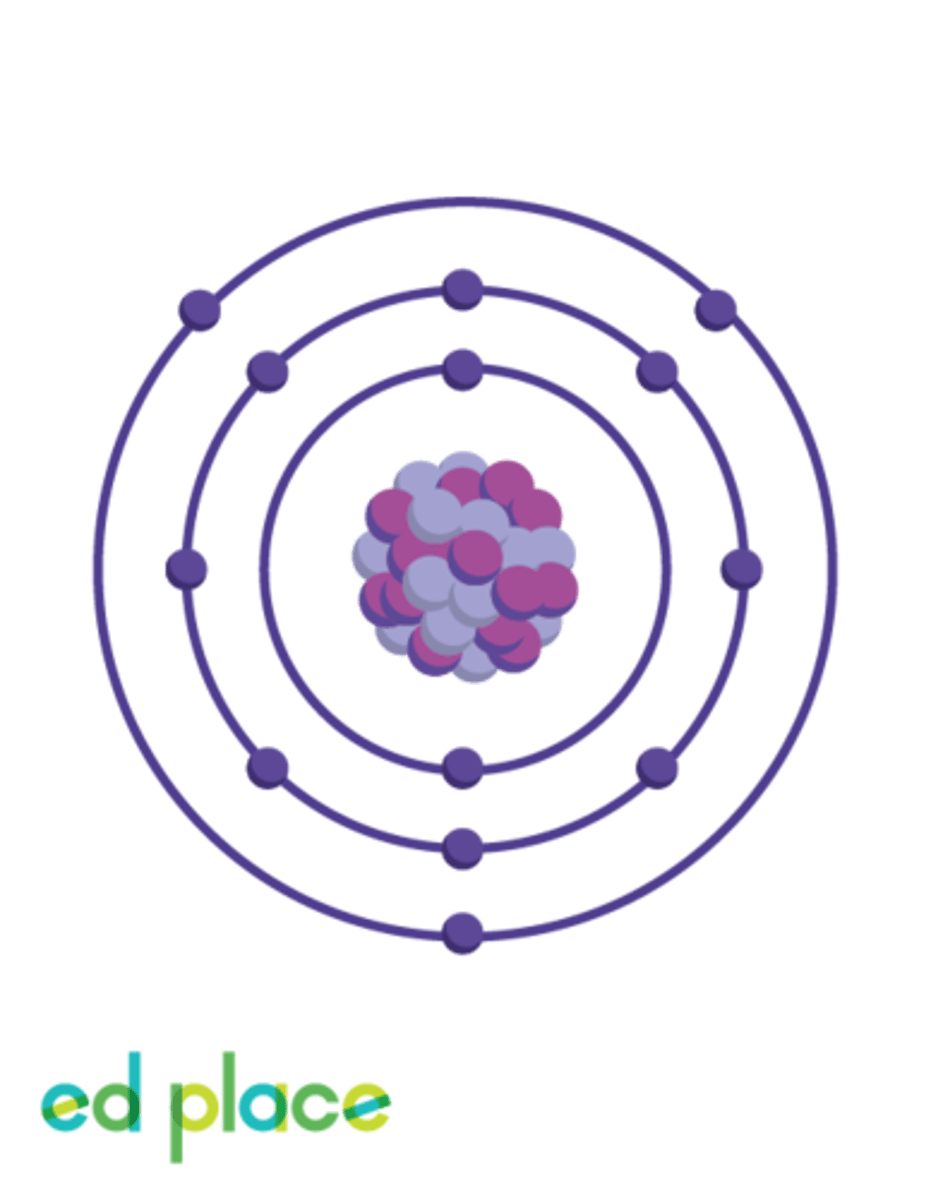
What is the atomic number of aluminium?
13