Chemical Kinetics
5.0(7)
5.0(7)
Card Sorting
1/18
Last updated 5:34 PM on 3/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
1
New cards
Kinetics
Study of the rates of reactions and the steps by which the occur
2
New cards
Endothermic
Pertaining to a chemical process that absorbs heat energy and produces positive change in enthalpy
3
New cards
Exothermic
Pertaining to a chemical process that releases heat energy and produces a negative change in enthalpy
4
New cards
Collision Theory Rule 1
Particles must collide before they can react. However, a collision alone doesn’t guarantee a reaction
5
New cards
Collision Theory Rule 2
Be properly oriented for the necessary rearrangement of atoms and electrons
6
New cards
Collision Theory Rule 3
Be forceful enough/possess enough energy to form products. Reactions follow only forceful, oriented collisions
7
New cards
Activation energy
Minimum amount of kinetic energy that must be possessed by the colliding molecules before they react
8
New cards
Activated complex
Colliding reactants that form a theoretical, transitional structure between reactant and product
9
New cards
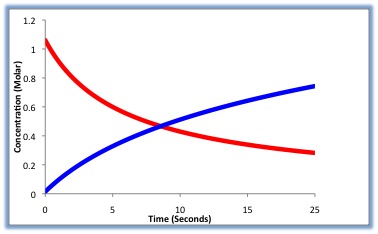
Reaction rates
tell how fast reactions change into products
10
New cards
Factors that affect reaction rates
Nature of reactants, concentration, temperature, surface area, and presence of catalysts
11
New cards
Nature of reactants
This controls the rate of the reaction. Reactive substances react quickly and vice versa. Some noble gases never react or barely do. Another variable is combination, H and Cl under certain conditions react vigorously.
12
New cards
Concentration
This increases the rate of a reaction. Varies from reaction to reaction, it could double or even quadruple the rate
13
New cards
Temperature
Reaction rates double for every 10 C in temperature. Higher temperatures increase number of collisions and the force of collisions
14
New cards
Surface area
If a substance is broken up into smaller pieces more surface area is exposed causing it to react faster.
15
New cards
Catalysts
A substance that changes a reaction rate without being permanently changed or consumed by the reaction. Has lower activation energy
16
New cards
Enzymes
A class of catalysts that are naturally occurring biological substances
17
New cards
Homogeneous catalyst
in the same phase as the reactants or in solution with a reactant
18
New cards
Heterogeneous catalyst
a catalyst in a separate phase than the reactant
19
New cards
Inhibitor
A substance used to reduce a catalysts undesirable effects.