Filarial Parasites: Morphology, Life Cycle, and Diagnosis
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
118 Terms
Dracunculus medinensis
Longest nematode parasite that infect man.
Common names of Dracunculus medinensis
Guinea worm, Serpent worm, Dragon worm, Medina worm, Fiery serpent of Israelites.
Larva of Dracunculus medinensis
Rhabditiform larvae, average size: 620 by 15 µm, coiled, rounded anterior end and tapering pointed tail.
Adult Dracunculus medinensis
Generally whitish and filament-like, primarily inhabit cutaneous and subcutaneous tissues.
Female Dracunculus medinensis
Prominent blunt, rounded anterior end, 840 mm long by 1.5 mm wide.
Male Dracunculus medinensis
Smaller than the female, measuring only 21 by 0.4 mm.
Pathogenesis of Dracunculus medinensis
Infection induces no illness till the gravid female worm comes to lie under the skin, ready to discharge its embryos.
Toxicity of adult Dracunculus medinensis
The body fluid of the adult worm is toxic and leads to blister formation.
Common sites for blister formation
The feet between the metatarsal bones or on the ankles.
Secondary bacterial infection
Frequent occurrence that may lead to tetanus.
Laboratory diagnosis of Dracunculus medinensis
Diagnosis is evident when the tip of the worm projects from the base of the ulcer.
Detection of larva
By bathing the ulcer with water, the worm can be induced to release the embryos.
Skin test for Dracunculus medinensis
An intradermal test with guinea worm antigen elicits a positive response.
Serological test for Dracunculus medinensis
Enzyme-linked immunosorbent assay (ELISA) and immunofluorescence assay (IFA) are frequently used to detect antibodies.
Infective stage of Dracunculus medinensis
L3 (filariform larvae).
Diagnostic stage of Dracunculus medinensis
Larva, adult worm.
Mode of transmission for Dracunculus medinensis
Drinking unfiltered water containing infected cyclops.
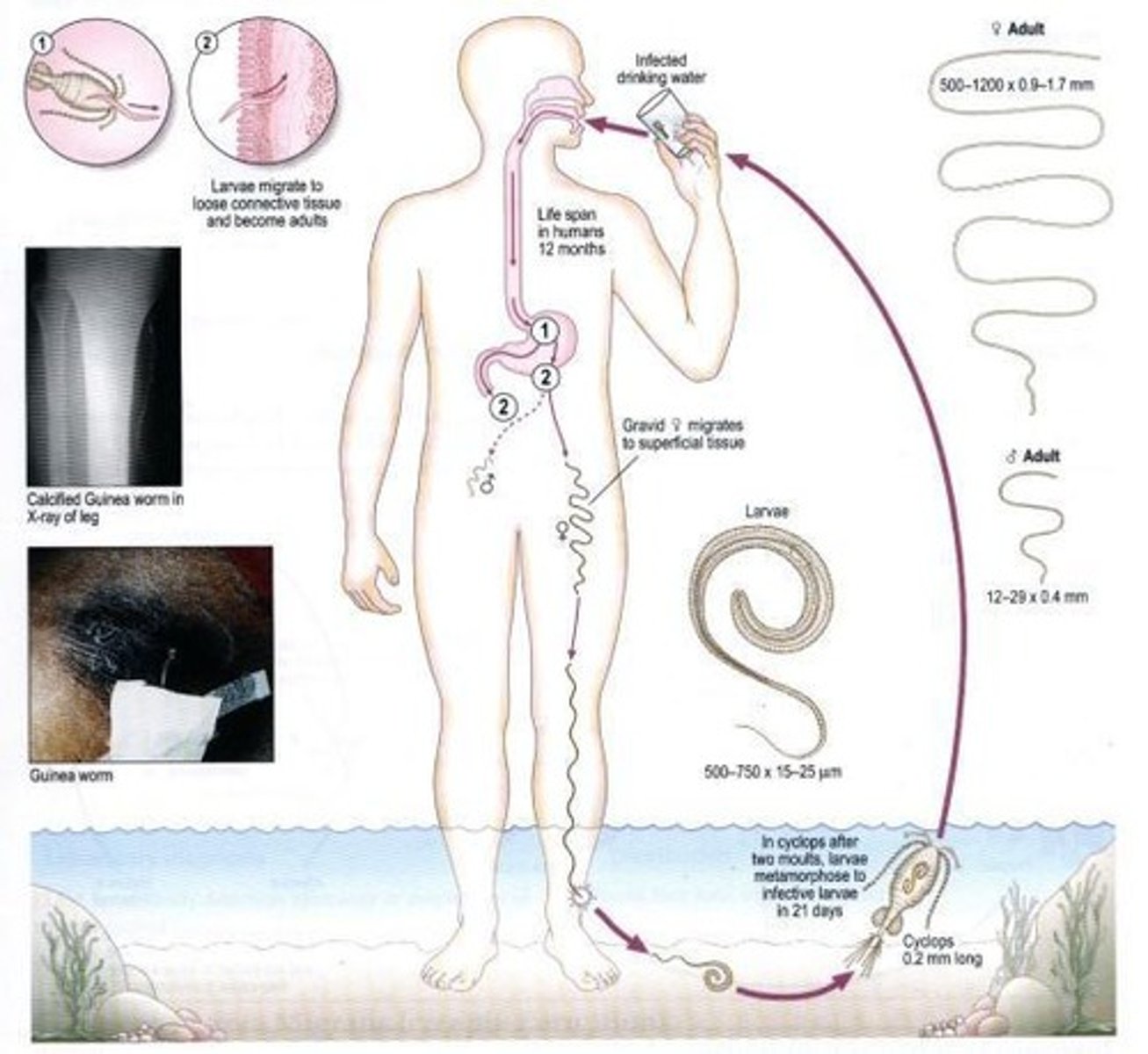
Intermediate host of Dracunculus medinensis
Cyclops (water fleas).
Prevention and control of Dracunculus medinensis
Use of properly treated water for consumption, boiling water suspected of contamination.

Filtering water for Dracunculus medinensis
Copepods may be removed from suspected water using a finely meshed filter.
Parastrongylus cantonensis
Common name: Rat Lungworm, first reported from Taiwan in 1945.
Egg of Parastrongylus cantonensis
Measure 46 to 48 μm by 68 to 74 μm and are unembryonated when oviposited.
Larva of Parastrongylus cantonensis
Found in the lungs of the rodent (L1), distinct small knob near the tip of the tail.
Adult Parastrongylus cantonensis
Generally pale and filiform/threadlike, male: 16-22 mm in length, female: 19-33 mm in length.
Female Parastrongylus cantonensis
Has the characteristic barber's pole pattern (twisting of intestines and uterus).
Gastropod intermediate host
They penetrate or are ingested by a gastropod intermediate host.
Third-stage larvae
After two molts, third-stage larvae are produced which are infective to mammalian hosts.
Definitive host
When the infected gastropod is ingested by the definitive host, the third-stage larvae migrate to the brain where they develop into young adults.
Young adults
The young adults return to the venous system and then the pulmonary arteries where they become sexually mature.
Paratenic hosts
Various animals act as paratenic (transport) hosts: after ingesting the infected snails, they carry the third-stage larvae which can resume their development when the paratenic host is ingested by a definitive host.
Human infection acquisition
Humans can acquire the infection by eating raw or undercooked snails or slugs infected with the parasite; they may also acquire the infection by eating raw produce that contains a small snail or slug, or part of one.
Larvae migration in humans
In humans, larvae migrate to the brain, or rarely to the lungs, where the worms ultimately die.
Development stages in humans
Larvae may develop to fourth or fifth stage in the human host, but seem to be incapable of maturing fully.
Natural host
Rats.
Intermediate hosts
Molluscs, slugs, and snails.
Infective form
Third stage larvae.
Mode of transmission
Human infection is acquired by eating infected mollusks and other intermediate hosts containing the third stage larvae.
Eosinophilic myelopencephalitis
Larva invades the eye and CNS.
Diagnosis of parastrongyliasis
Diagnosis of parastrongyliasis in humans is relatively difficult, since the primary site of infection is the brain.
Presumptive diagnosis
Presumptive diagnosis may be made based on travel and exposure history, correlated with clinical symptoms, medical history, laboratory findings, brain imaging results, and serological tests.
Eosinophils count
Eosinophils comprising 7 to 36% of the white blood cell (WBC) count.
Cerebrospinal fluid (CSF) protein level
CSF protein level in most patients is mildly elevated.
CSF glucose
CSF glucose is normal.
CT scans
CT scans may also reveal non-specific cerebral edema and ventricular dilatation.
Magnetic resonance imaging (MRI)
MRI may show lesions with hyperintense T2 signal.
Serology
Dot-blot ELISA that tests blood has been demonstrated to be 100% sensitive and specific for use in epidemiological surveys.
Serum antigens detection
Serum antigens from P. cantonensis can also be detected by immunopolymerase chain reaction (PCR).
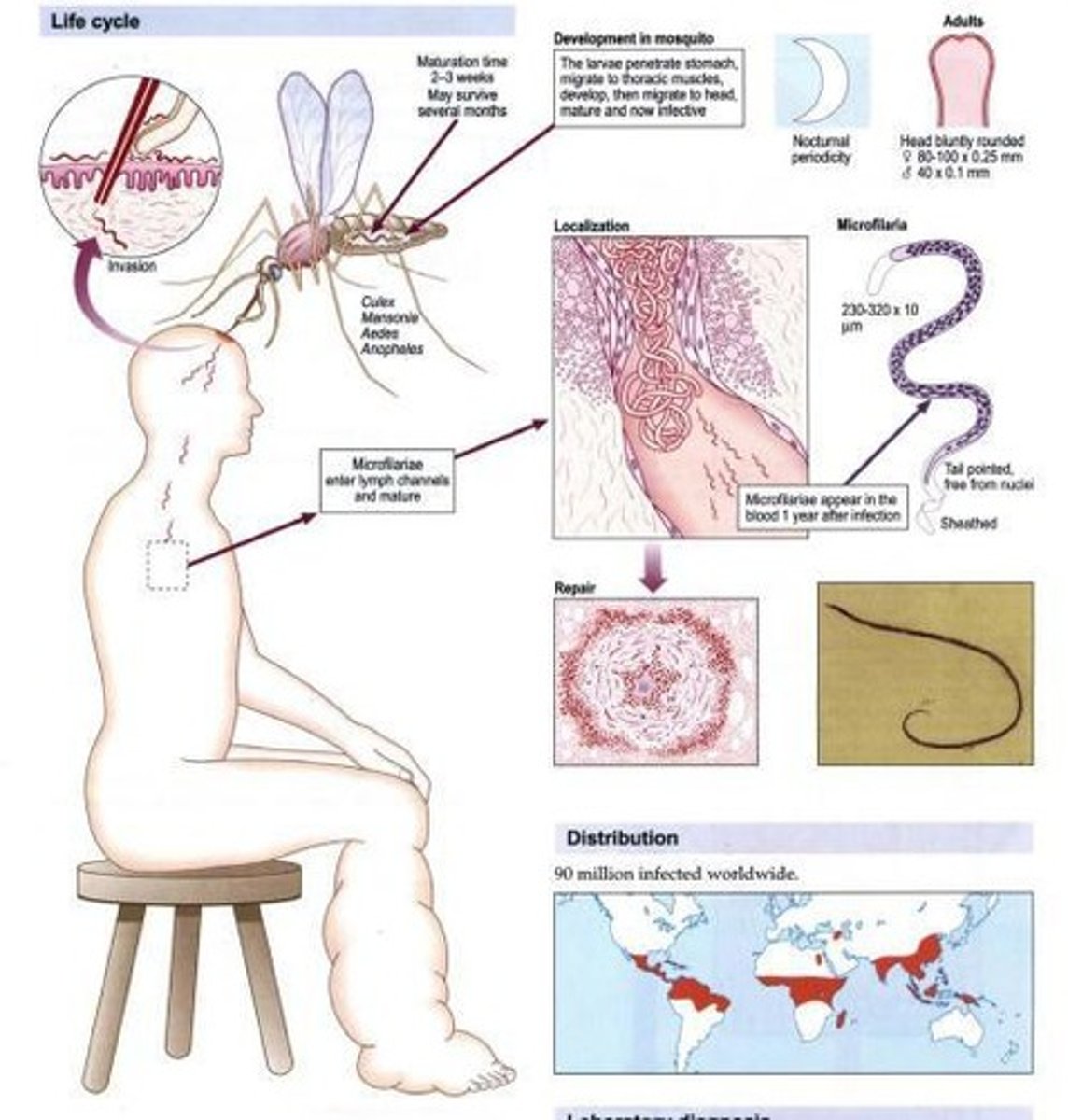
Wuchereria bancrofti
Other name: Bancroft's filaria.
Habitat of Wuchereria bancrofti
Lymphatics.
Vector of Wuchereria bancrofti
Mosquito (Anopheles, Culex, Aedes).
Disease caused by Wuchereria bancrofti
Elephantiasis (lower) with chylocoele/hydrocoele and chyluria, Tropical Pulmonary eosinophilia.
Prevention and control measures
Awareness and education on proper eating habits and safe food preparation; washing after gardening should also be advised; molluscicides.
Gnathostoma spinigerum
Rust-colored, cephalic bulb with four rows of hooks.
MOT of Gnathostoma spinigerum
Ingestion of larva in fish, birds and snakes.
Disease caused by Gnathostoma spinigerum
Gnathostomiasis, visceral and cutaneous larva migrans-like syndrome, CNS improvement (Eosinophilic myelopencephalatis).
Microfilaria size
Measures 270 - 290 um.
Microfilaria concentration timing
Highest concentration in nighttime, about 10pm to 2am.
Dirofilaria immitis
Commonly known as 'Dog heartworm', it is a filarial parasite that is unsheathed and has partial nocturnal periodicity.
Anisakis
'Herring's worm', a parasite with whales and dolphins as definitive hosts and three intermediate hosts: copepods, small fishes, and larger fishes.
Filarial Parasite
Refers to eight known species of filarial nematodes that use humans as their definitive host.
Microfilariae
Sheathed larvae produced by adult filarial worms that have nocturnal periodicity and migrate into lymph and blood channels.
Life Cycle of Filarial Parasites
Involves transmission by mosquito bites, development in lymphatics, and migration of microfilariae to infect another host.
Laboratory Diagnosis of Filarial Infection
Depends on clinical features, history of exposure, and laboratory findings, with peripheral blood being the specimen of choice.
Biopsy in Filarial Diagnosis
Adult filarial worms can be seen in sections of biopsied lymph nodes, though this is not routine.
Skin Test for Filarial Infection
Involves intradermal injection of filarial antigens to test for infection.
Imaging Techniques for Filarial Diagnosis
Ultrasonography and radiology are used in the diagnosis of filariasis.
Xenodiagnosis
A method where mosquitoes feed on infected patients and are dissected later to demonstrate microfilariae.
Preventive Measures for Filarial Infection
Includes personal protective measures, mosquito nets, and insecticide residual spraying to reduce mosquito vectors.
Pathogenesis of Filarial Infection
Describes the asymptomatic presence of microfilariae, acute symptoms like fever and lymphadenitis, and chronic conditions like lymphedema.
Acute Stage of Filarial Infection
Characterized by early manifestations including fever and inflammation of lymph glands (lymphadenitis).
Chronic Stage of Filarial Infection
Involves chronic pitting edema progressing into non-pitting edema, with potential for lower elephantiasis.
Adult Male Filarial Worms
About 20 - 40 mm in length and 100 um in width, with a ventrally curved tail and 15 pairs of caudal sensory organs.
Adult Female Filarial Worms
80-100 mm in length and 300 um in width, characterized by anterior vulva and tapered tails.
Filarial Nematodes
Slender thread-like worms transmitted by the bite of blood-sucking insects.
Granulomatous Abscess
Disease caused by Anisakis, characterized by abscesses in the gastric and intestinal mucosa.
Coin Lesion
Solitary, peripheral nodules in the lung associated with Dirofilaria immitis infection.
Intermediate Hosts of Anisakis
Include copepods as the first, small fishes as the second, and larger fishes as the third.
Ingestion of Raw Fish
Method by which humans acquire Anisakis infection.
Lymphatic Development
Filarial larvae develop into adults that commonly reside in the lymphatics of the human host.
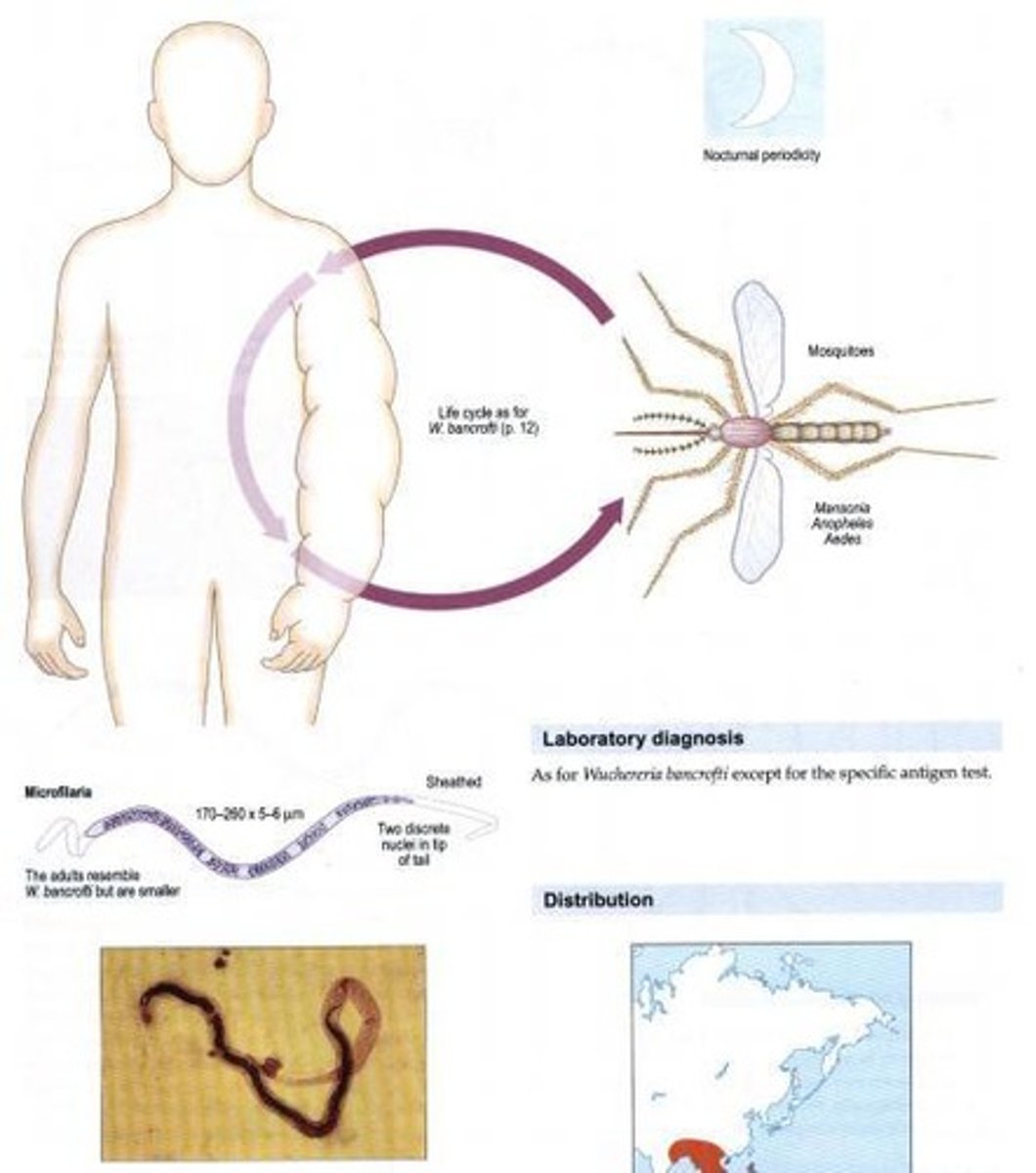
Brugia malayi
Other name: Malarian filaria. Habitat: Lymphatics. Vector: Mosquito (Mansonia). Disease: Elephantiasis (upper) with chylocoele/hydrocoele and w/o chyluria, Immunosuppression inducing apoptosis of CD4+ cells.
Dreyer's staging for chronic lymphedema
Stage 1: Swelling increases in day but disappears overnight (especially when lying in bed). Stage 2: Swelling no longer reversible, occurrence of acute attacks and can have bad odor. Stage 3: Presence of shallow skin folds, occasional acute attacks and bad odor. Stage 4: Knobs and protrusions are found in skin, occasional acute attacks and bad odor. Stage 5: Deep skin folding, frequent acute attacks and lymphedema can go beyond the knee. Stage 6: Mossy lesions (mossy foot), acute attacks and wounds in skin. Stage 7: Patient cannot perform daily tasks independently anymore.
Microfilaria of Brugia malayi
Measures 111 - 230 um, enclosed in sheath and with 2 nuclei at tip of tail: inconspicuous rows of nuclei.
Adult Brugia malayi
Males (13-23 mm length, 70-80 um in width) and females (43-55 mm in length, 130-170 um in width). Generally, resemble Wuchereria bancrofti but are smaller.
Laboratory diagnosis for Brugia malayi
Examination of stained blood films serves as the best method for diagnosis.
Prevention and control of Brugia malayi
Personal protective measures may help prevent contact with mosquito vectors. The use of mosquito nets as well as insecticide residual spraying advances in vector control. Polystyrene beads to seal latrines in order to eliminate or reduce Culex vector. Health education.
Treatment for Brugia malayi
Diethylcarbamazine.
Onchocerca volvulus
Other name: Convoluted filaria, Blinding worm. Habitat: Subcutaneous tissue. Vector: Blackfly/Buffalo gnat (Simulium damnosum). Disease: Blinding filariasis, River blindness, Onchocercomata, Hanging groin, Leopard skin.
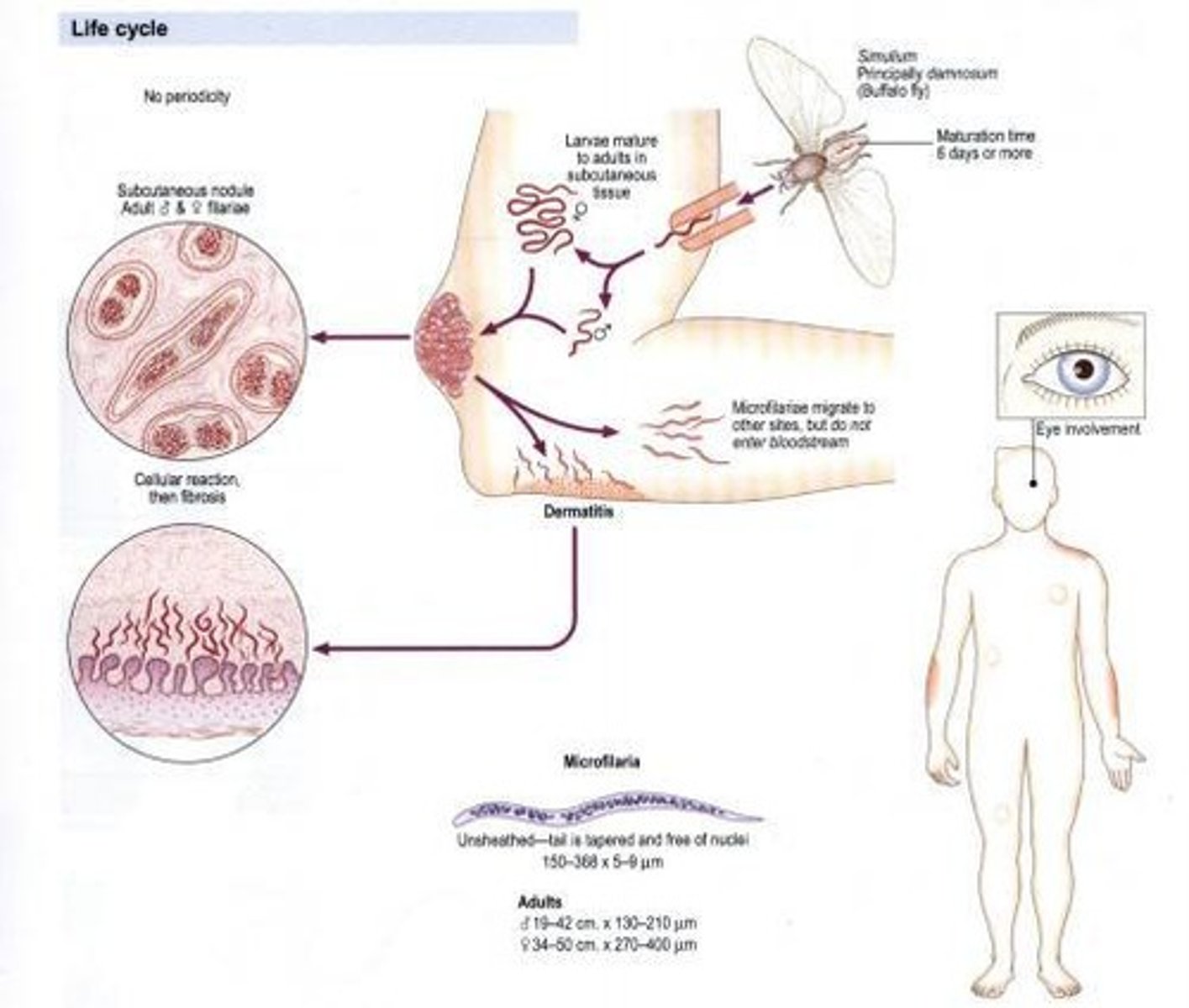
Microfilaria of Onchocerca volvulus
220-360 um in length and 5-9 um in width, can persist for 2 years in host, has a large cephalic space, nuclei around body are well separated with no nuclei found at pointed tail. Usually deposited in eyes, skin or lymph nodules; tail is flexed or tapered, unsheathed.
Adult Onchocerca volvulus
Males (19-42 mm in length, 130-210 um in width) and females (33-50 cm in length, 270-400 um in width). Wirelike and whitish in appearance.
Life cycle of Onchocerca volvulus
1. During a blood meal, an infected mosquito (typically Mansonia spp. and Aedes spp.) introduces third-stage filarial larvae onto the skin of the human host, where they penetrate into the bite wound. 2. They develop into adults that commonly reside in the lymphatics. The adult worms outwardly resemble those of Wuchereria bancrofti but are smaller. 3. The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood. 4. A mosquito ingests the microfilariae during a blood meal. 5. After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles. 6. There the microfilariae develop into first-stage larvae and subsequently into third-stage larvae. 7. The third-stage larvae migrate through the hemocoel to the mosquito's proboscis and can infect another human when the mosquito takes a blood meal.
Pathogenesis of Onchocerca volvulus
Usually involves UPPER lymphatics; B. malayi can potentially suppress T cell functions.
Loa loa
Other name: African eye worm. Habitat: Subcutaneous tissue. Vector: Mango/Deer/Tabanid fly (Chrysops). Disease: Calabar/Fugitive swelling.
Life cycle of Loa loa
1. During a blood meal, an infected blackfly introduces third-stage filarial larva onto the skin of the human host, where they penetrate into the bite wound. 2. In subcutaneous tissues the larvae develop into adult filariae, which commonly reside in nodules in subcutaneous connective tissues.
Adult filariae
Can live in nodules for approximately 15 years.
Microfilariae
Ingested by blackfly during a blood meal and migrate to thoracic muscles.
Third-stage infective larvae
Migrate to the blackfly's proboscis and can infect another human when the fly takes a blood meal.
Onchocerciasis
Causes lizard/leopard skin, hanging groin, localized onchocercal dermatitis, and keratitis.
Mazzoti skin test
Topical application of 1.6% DEC leads to local reaction, or initial 50 mg DEC is given orally.
Ivermectin
Used for treatment of filarial infections.
Mansonella spp.
Vector is culicoides/midge; definitive hosts are humans.