
Looks like no one added any tags here yet for you.
molecular orbitals
likelihood of finding electrons in space around molecule
resonance structures
used to describe delocalization of pi electrons in molecule
hydrocarbons
unreactive, primarily function as scaffold
saturated
hydrocarbons that contain only single bonds between carbon atoms.
unsaturated
Containing one or more double or triple bonds between carbon atoms
heteroatom
An atom in an organic molecule that is not carbon or hydrogen
intermolecular forces result
charge interactions
electrostatic interactions
that arise from the attraction or repulsion between charged particles or polar molecules, full formal charges, strongest IMF
dipole-dipole
interactions that occur between polar molecules due to the attraction of positive and negative ends
hydrogen bonding
a strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine.
London dispersion
forces are weak intermolecular forces that arise from temporary shifts in electron density in non-polar molecules
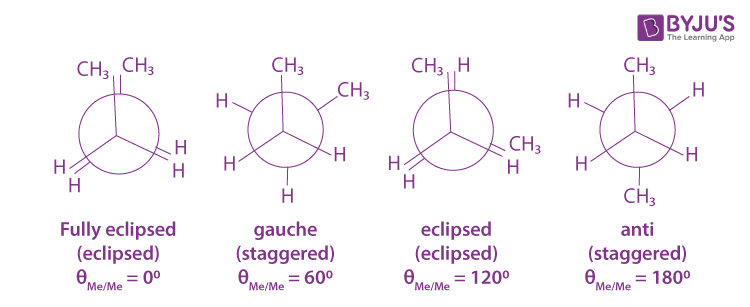
conformations
different spatial arrangements of a molecule that can occur due to rotation around single bonds.
torsional strain is minimum in
staggered conformation
staggered conformation
front and back groups are as far apart as possible, least torsional strain therefore lowest energy
steric strain
repulsive force between the electron clouds of atoms that are close to each other nut not directly bonded
angle strain
bond angles that do not permit maximum orbital overlap between atoms of a molecule
equatorial position
the position in a cyclohexane ring where substituents are located in the plane of the ring—>chair conformer
constitutional isomers
same molecular formula, different connectivity
stereoisomers
same molecular formula, connectivity, different arrangement of atoms in space
enantiomers
stereoisomers that are non-superimposable mirror images (chiral)
chirality
non-superimposable on their mirror image
diastereoisomer
stereoisomers that are non-superimposable, non-mirror images
meso compounds
molecules that contain more than 1 stereocenter and have superimposable mirror images
how does resonance effect organic molecules
greatly stabilized by the delocalization of pi electrons
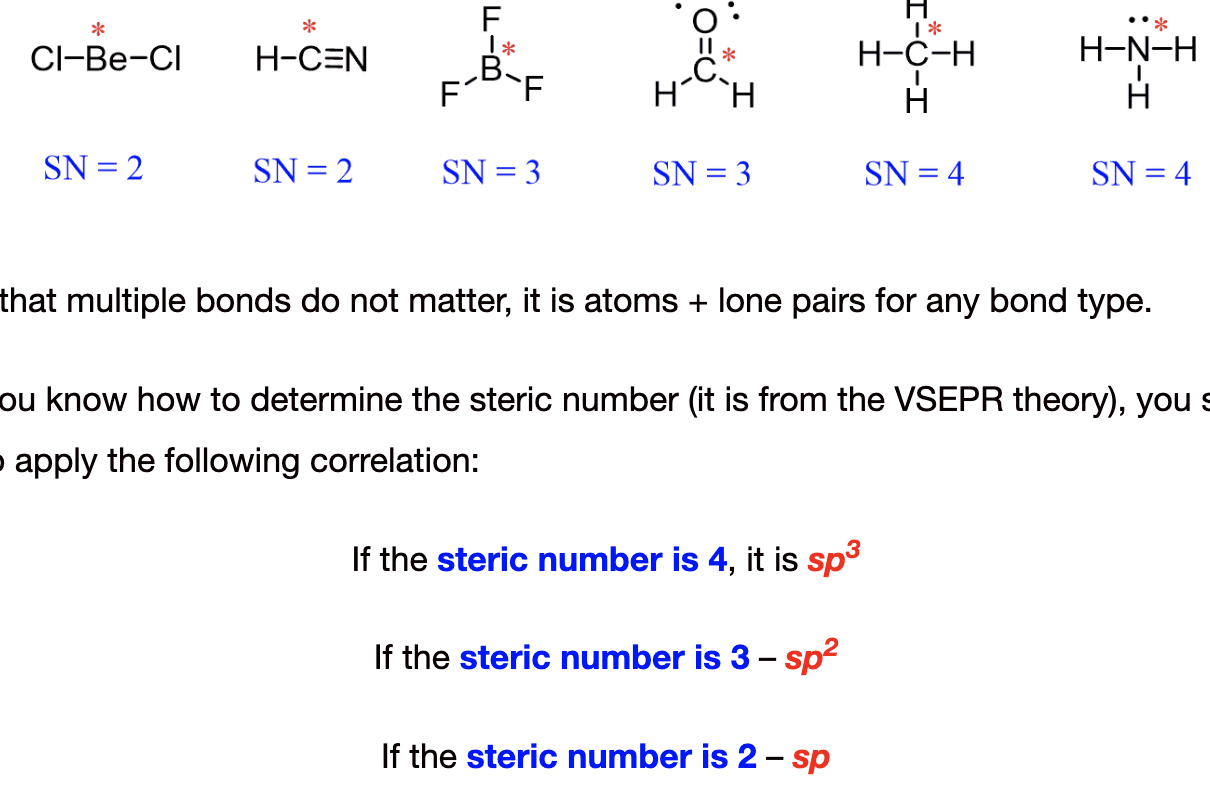
hybridization
of atomic orbitals to form new hybrid orbitals that can accommodate bonding (sp2, etc)
formal charge
formal charge= group# - #of bonds- #of non-bonded electrons
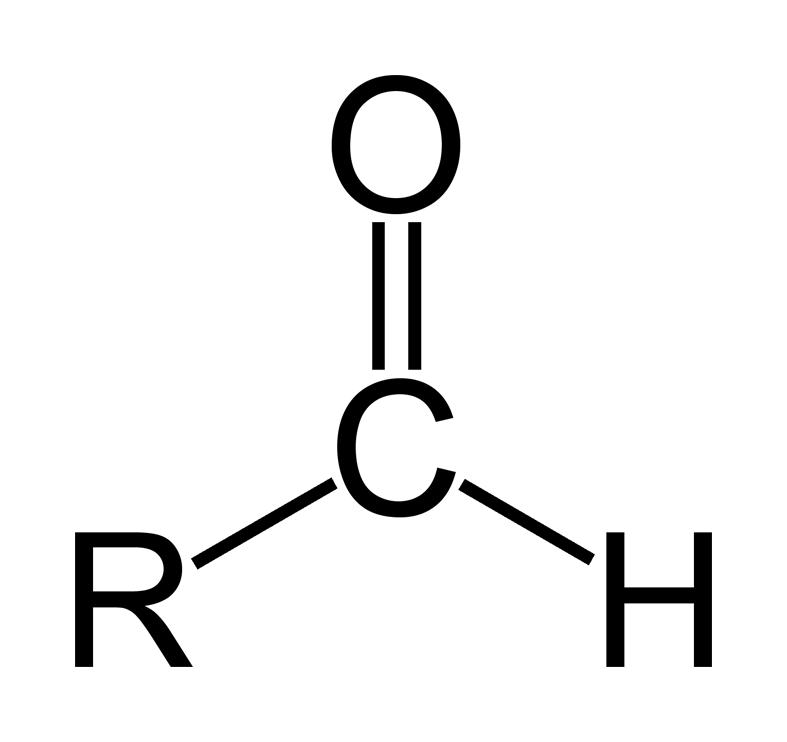
aldehyde
alkanes
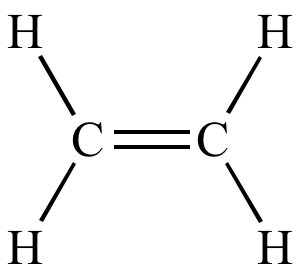
alkenes
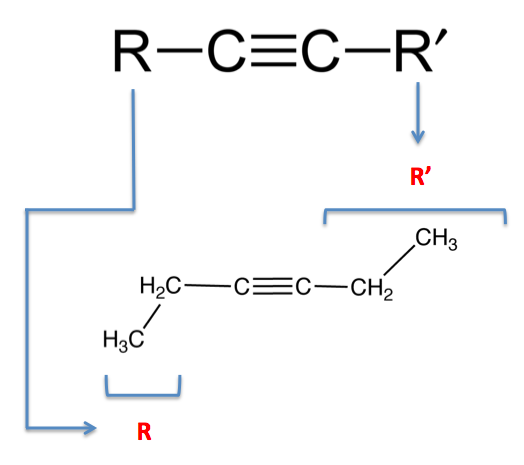
alkynes
aromatic rings
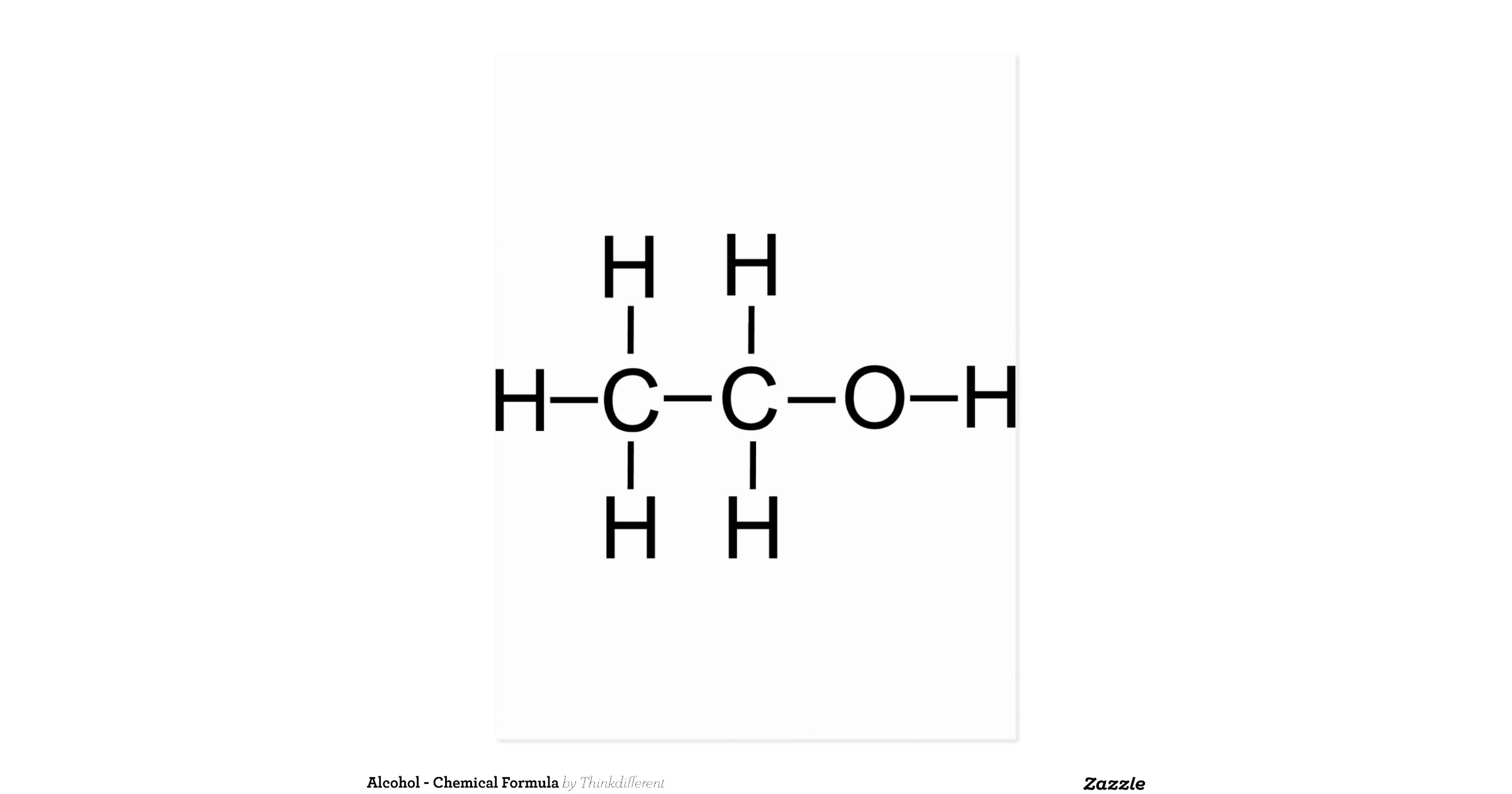
alcohols
ethers

aldehydes
ketones
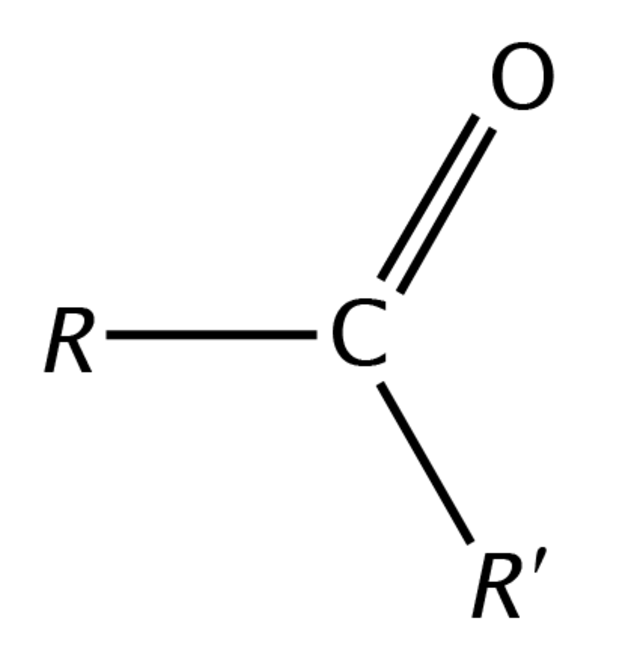
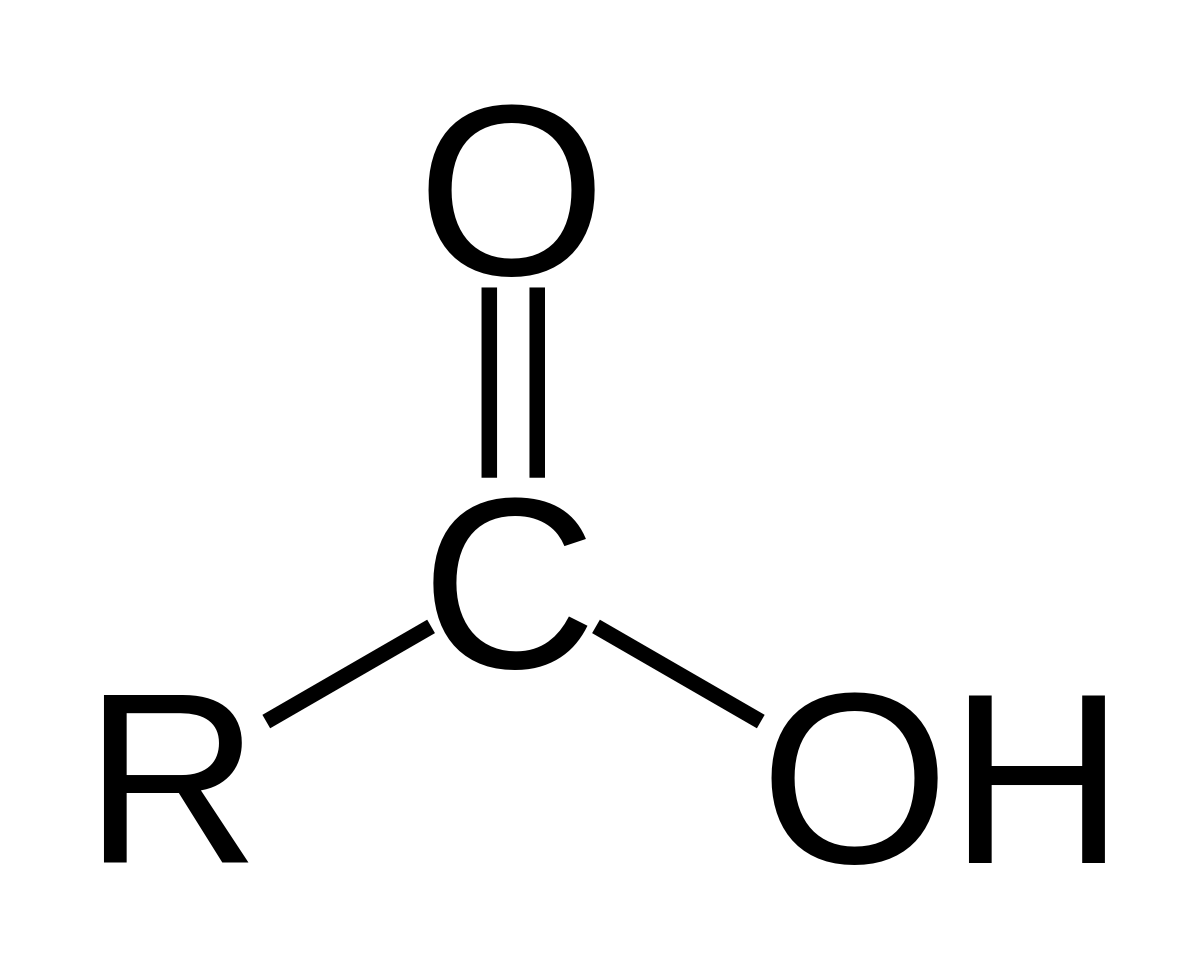
carboxylic acid
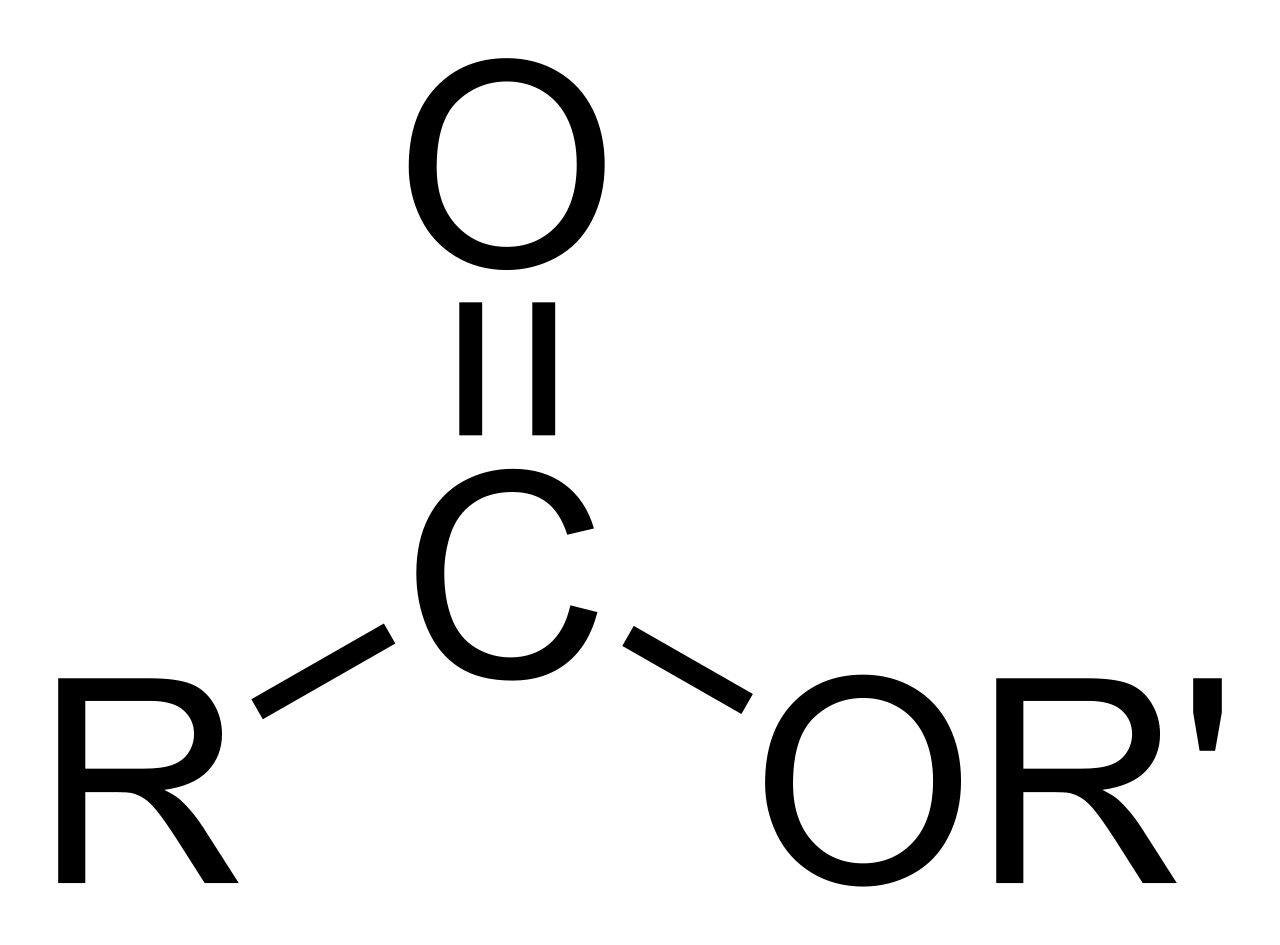
esters
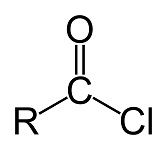
acid halides
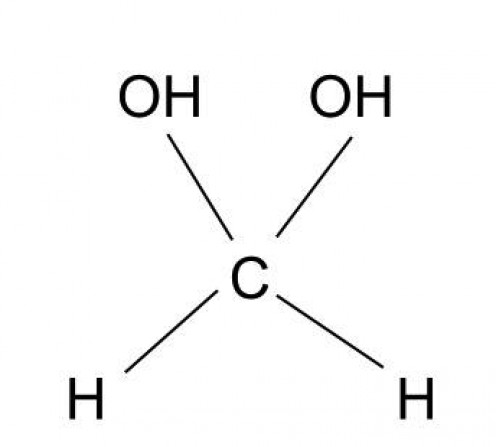
hydrates
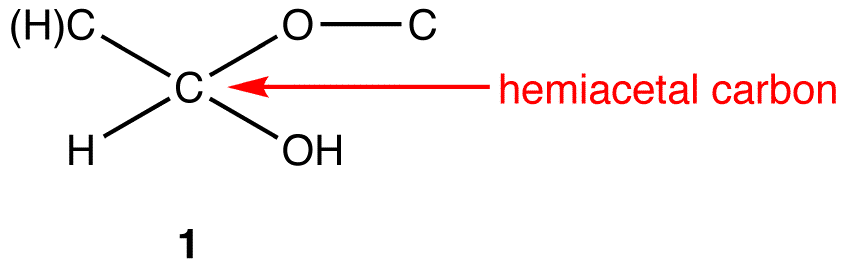
hemiacetals
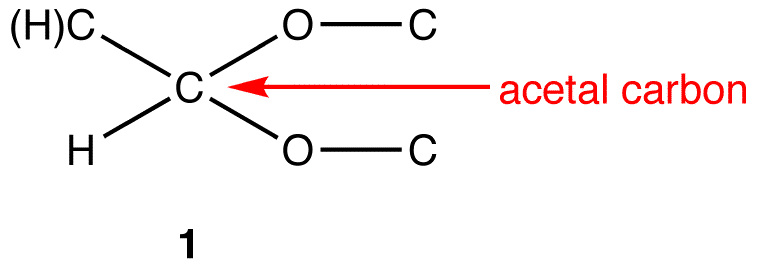
acetals
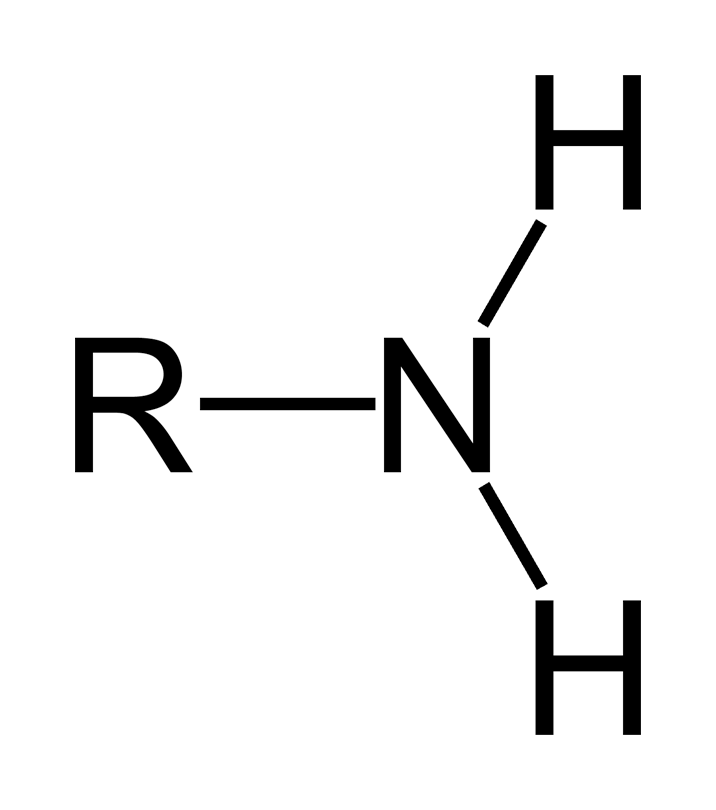
amines
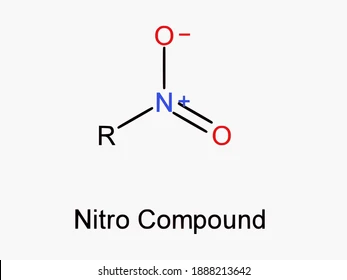
nitros
nitriles

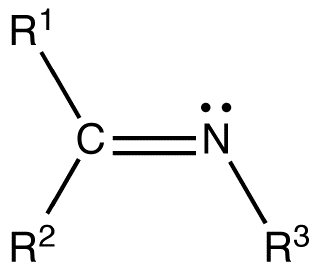
imines
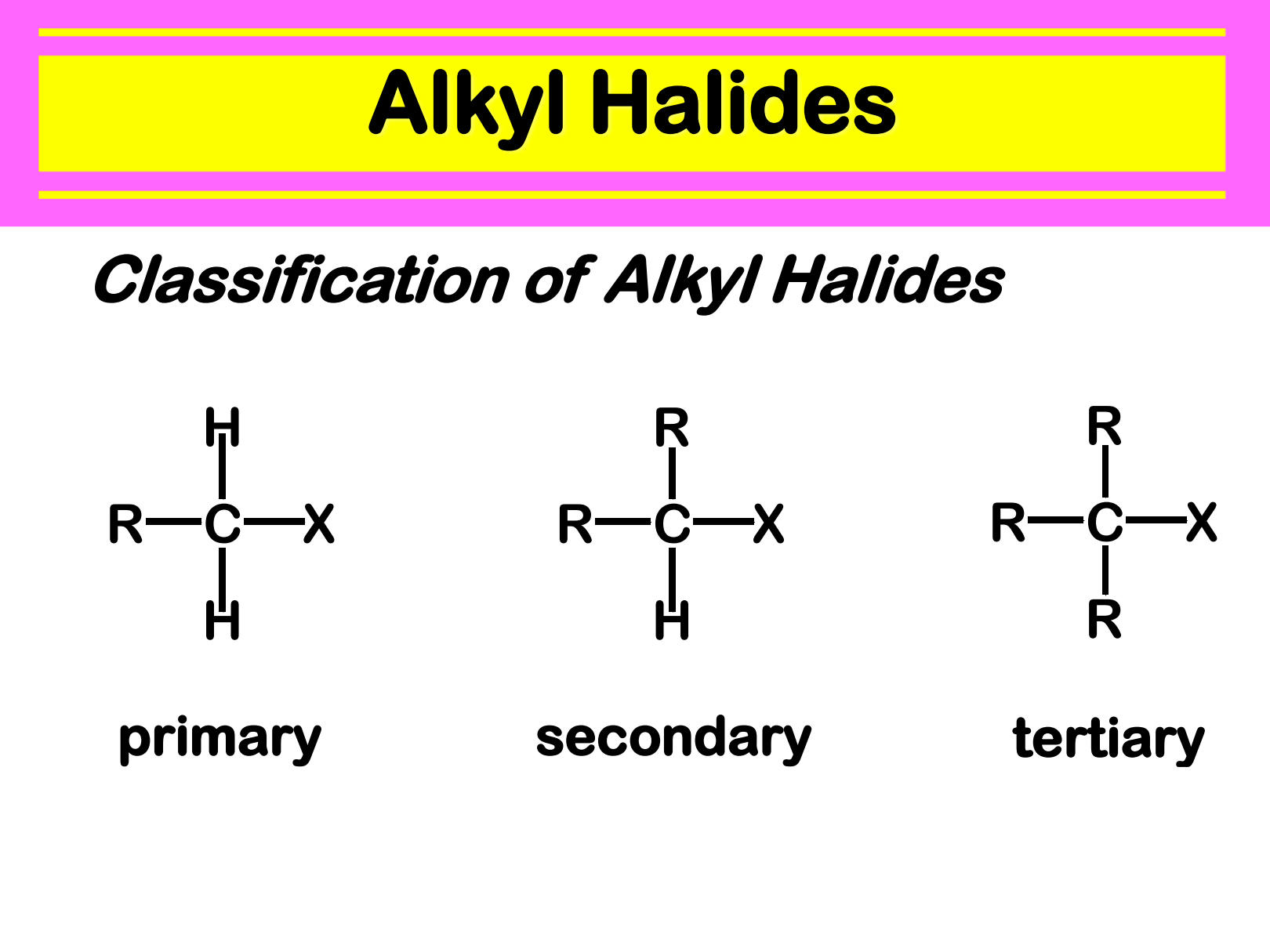
halides
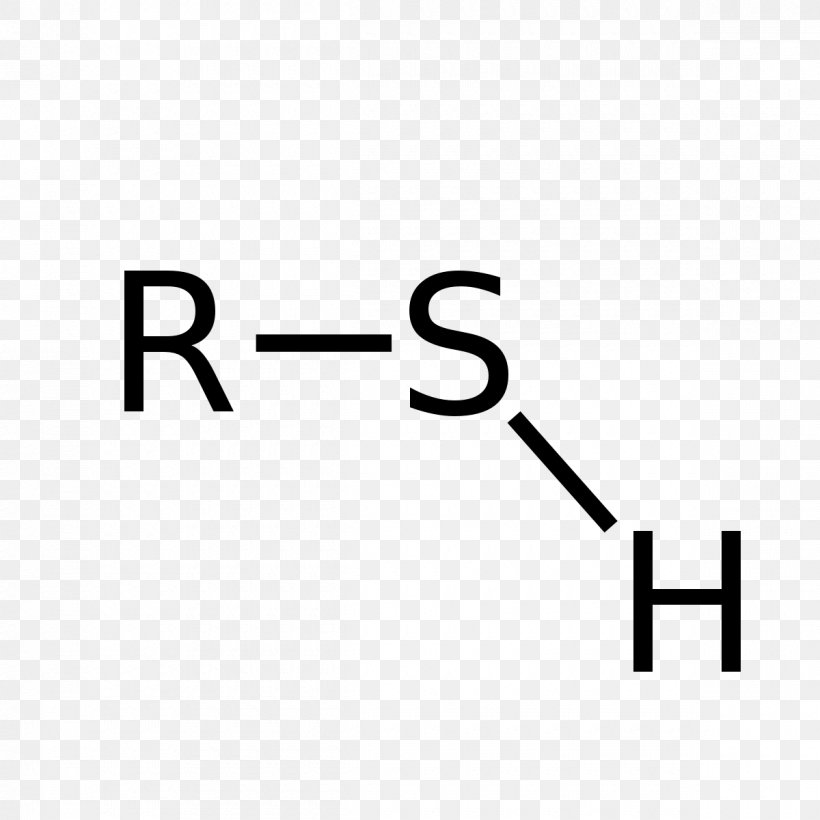
thio-
polar
A molecule with a distribution of electric charge, resulting in a positive and negative end. Have electronegativity
non-polar
A molecule that has an even distribution of electric charge, resulting in no distinct positive or negative ends.
axial and equatorial groups
axial: chair six groups pointing directly up/down
equatorial: chair six groups pointing outwards
achiral
A molecule that cannot be superimposed on its mirror image, indicating it has no chiral centers.
stereogenic centre
A carbon atom bonded to four different substituents, leading to non-superimposable mirror images.
R/S descriptors
stereogentic centre, finding way of rotation via priority around centre
R= right/clock wise
S= left/counterclockwise
meso
A compound with multiple stereogenic centers that is superimposable on its mirror image, resulting in no optical activity.
E/Z configurations
E indicates higher priority groups are on opposite sides, while Z indicates they are on the same side.
sigma bond
A type of covalent bond formed by the direct overlap of atomic orbitals, allowing for free rotation around the bond axis.
pi bond
A type of covalent bond formed by the lateral overlap of p-orbitals, allowing for electron sharing between atoms. Pi bonds are typically found in double and triple bonds.
How to use IUPAC naming
determine principal characteristic group
identify parent chain
choose appropriate root word
identify double or triple bonds
identify side chains or lower functional groups
assign E/Z system
how to show overall transformation
number different steps around the same arrow
types of arrows
—> irreversible
<==> reversible
<=—> biased reversible
←→ relates resonance structures
nucleophile
donates an electron pair to form a chemical bond in a reaction, typically seeking positively charged or electron-deficient sites.
electrophile
accepts an electron pair from a nucleophile during a chemical reaction, often possessing a positive charge or a partial positive charge.
what does a reaction coordinate show
relative energies of species in a reaction
define starting materials, intermediates, and products
Starting materials are the reactants at the beginning of a reaction, intermediates are transient species formed during the reaction, and products are the final substances produced at the end.
transition states
what compound(s) look like while undergoing transformation
activation energy
energy difference between starting material/intermediate and transition state for specific reaction
exergonic
lower in energy than the starting materials
endergonic
higher in energy than the starting materials
electron delocalization
large amount of stabilization, more resonance forms the more stable
Factors of importance for resonance
full octets
fewest formal charges
negative formal charges on electronegative
How to identify sigma and pi bonds
1 single=1s
1 double= 1s and 1p
1 triple= 1s and 2p
How to do sp
butane conformational
Superimposable
structures that can be overlaid on each other without any differences in their arrangement.