5. Electrochemical gradients and equilibrium potentials
1/8
Earn XP
Description and Tags
Lecture 5
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Which ions are most important in establishing membrane potential, and how do they move at rest?
Key ions: Na⁺, K⁺, Cl⁻
Concentrations differ inside vs. outside cell → creates membrane potential/voltage (Vm)
At rest: ions move via leak channels + transporters
Na+/K+ pump uses ATP to put 2 K+ in and 3 Na+ come out
At rest: Higher concentration of K+ inside, higher concentration of Na+ outside

What is the resting membrane potential and what causes it?
Voltage difference across excitable cell membrane at rest
Caused by uneven ion concentrations across membrane
Which factor mostly determines the resting membrane potential?
A) The leak of sodium ions across the cell membrane
B) The leak of potassium ions across the cell membrane
C) The sodium-potassium pump
D) None of the above
B) Potassium leak channels
K⁺ leak dominates because membrane is most permeable to K⁺ at rest
Na⁺ leak and Na⁺/K⁺ pump contribute but less directly
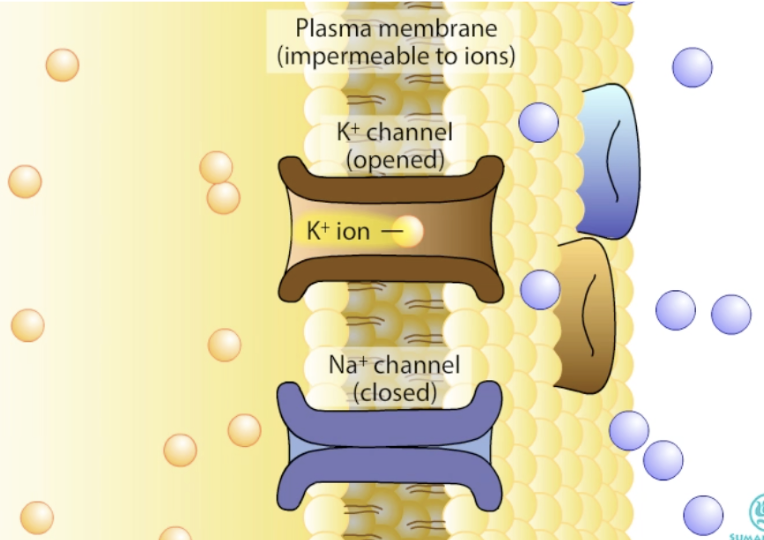
Why is the resting potential mainly determined by K⁺?
Many open K⁺ leak channels at rest → high permeability to K⁺
Thus, determines the resting potential
Na⁺/K⁺ pump maintains the potential at rest
Resting Vm ≈ EK (potassium equilibrium potential)
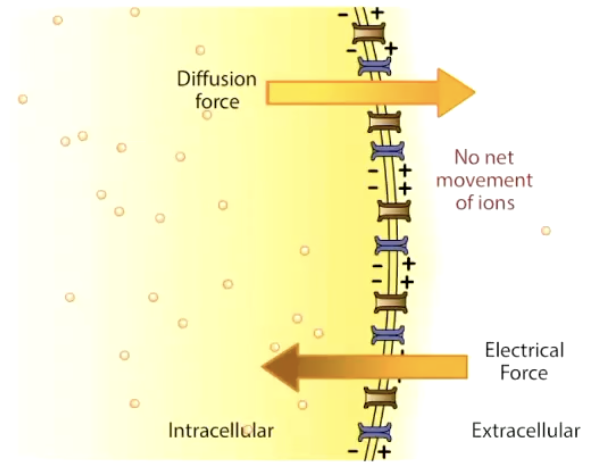
What is the electrochemical gradient and what is the goal of ion movement?
Combination of chemical (concentration) + electrical (voltage) forces
Ions move until equilibrium potential (Eion) is reached → no net movement
Equilibrium potential: The potential at which the electric part of the electrochemical force completely balances the concentration part of the electrochemical force
Until there is NO NET movement of that ion
How do you calculate equilibrium potential for an ion?
Nernst Equation
Where:
R = 8.314 J/(mol·K)
T = temp (Kelvin = °C + 273)
z = ion charge
F = 96,485 C/mol (charge of 1 more of electrons)
Steps:
Plug in [ion]o and [ion]i
Divide outside ÷ inside
Take log₁₀ of ratio
Multiply by 58 mV
Divide by valence (z)
![<p><strong><u>Nernst Equation</u></strong></p><p>Where:</p><ul><li><p>R = 8.314 J/(mol·K)</p></li><li><p>T = temp (Kelvin = °C + 273)</p></li><li><p>z = ion charge</p></li><li><p>F = 96,485 C/mol (charge of 1 more of electrons)</p></li></ul><p><strong><u><br>Steps:</u></strong></p><ol><li><p>Plug in [ion]o and [ion]i</p></li><li><p>Divide outside ÷ inside</p></li><li><p>Take log₁₀ of ratio</p></li><li><p>Multiply by 58 mV</p></li><li><p>Divide by valence (z)</p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/8d9f56d3-81bd-4dbe-b43c-11fd98b7b0c8.png)
What are the equilibrium potentials for Na⁺ and K⁺ in a typical mammalian cell?
Given:
[Na⁺]i = 15 mM, [Na⁺]o = 145 mM
[K⁺]i = 150 mM, [K⁺]o = 4 mM
Using Nernst (E = 58 log([out]/[in]))
Na⁺: E = 58 log(145/15) = +57 mV
K⁺: E = 58 log(4/150) = –91 mV
Why it matters: Eion values predict how ions drive changes in excitable cells during signaling. Cells are excited when the cell permeability changes to one/more ions.
For assignments, show your work and write out the equation!
![<p><strong>Given:</strong></p><ul><li><p>[Na⁺]i = 15 mM, [Na⁺]o = 145 mM</p></li><li><p>[K⁺]i = 150 mM, [K⁺]o = 4 mM</p></li></ul><p><strong>Using Nernst (E = 58 log([out]/[in]))</strong></p><ul><li><p>Na⁺: E = 58 log(145/15) = +57 mV</p></li><li><p>K⁺: E = 58 log(4/150) = –91 mV</p></li></ul><p><strong>Why it matters:</strong> Eion values predict how ions drive changes in excitable cells during signaling. Cells are excited when the cell permeability changes to one/more ions.</p><p></p><ul><li><p>For assignments, show your work and write out the equation!</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/14d7e6b8-5050-4559-b642-62b51d3a4a51.png)
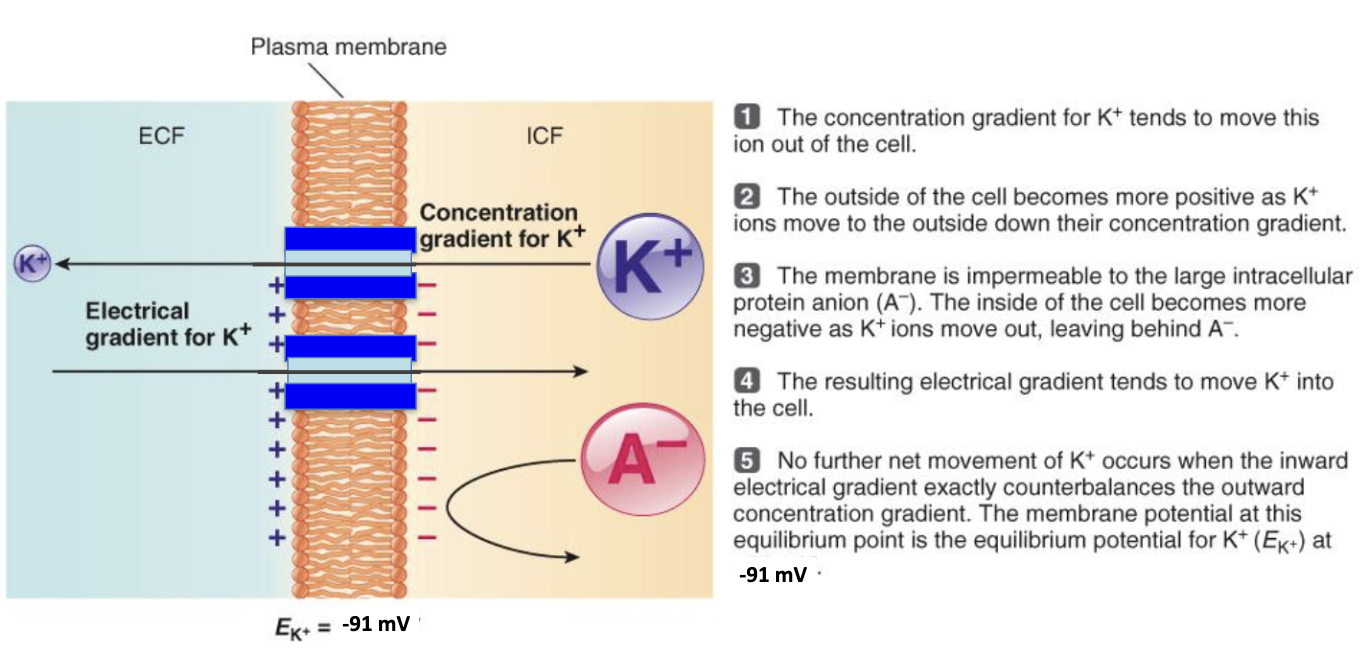
How is EK (–91 mV) reached for K⁺?
Conc. gradient pushes K⁺ out
Outside becomes +, inside – (due to trapped A⁻ proteins)
Electrical gradient pulls K⁺ back in
At –91 mV, forces balance → no net K⁺ movement
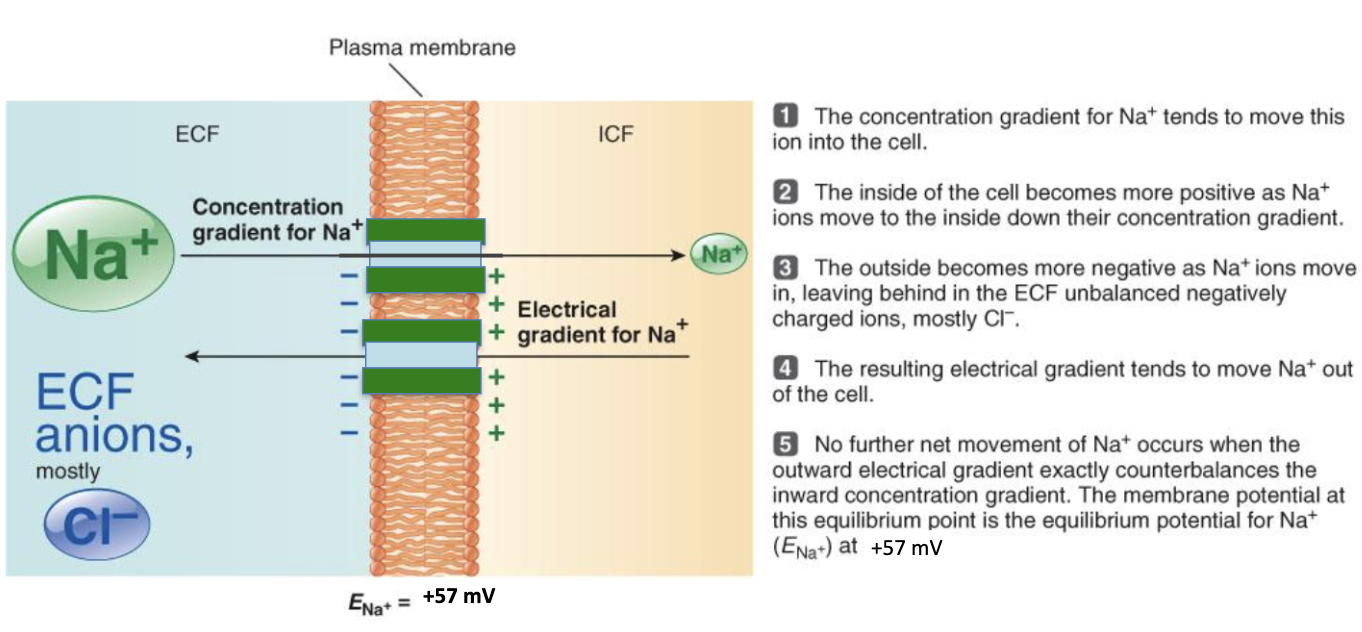
How is ENa (+57 mV) reached for Na⁺?
Conc. gradient pushes Na⁺ in
Inside becomes +, outside – (Cl⁻ left behind)
Electrical gradient pushes Na⁺ back out
At +57 mV, forces balance → no net Na⁺ movement