13 - Molecular Mechanisms of Cancer
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
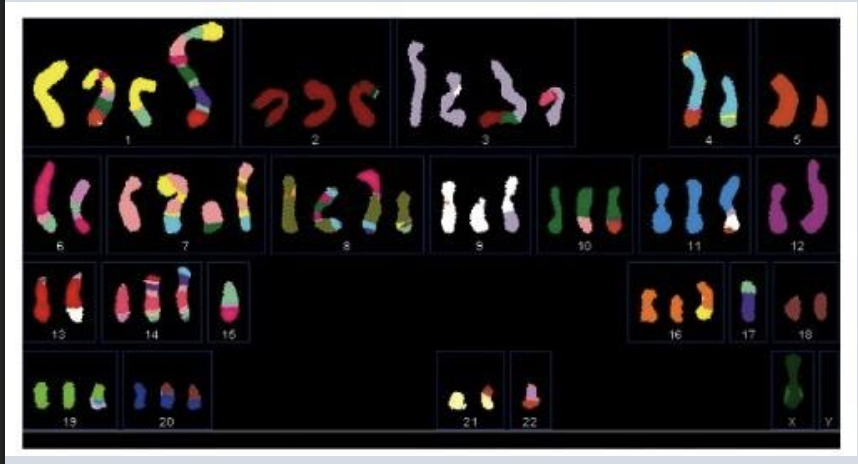
Based on this figure and your prior knowledge from the course, which of the following do you conclude:
A) The majority of human genomic DNA encodes biologically important information that cannot be disrupted in living cells
B) This cancer is caused by a mutation inherited from the patient’s parent
C) In the pancreatic cancer shown here, gene expression levels could be influenced by chromosome loss, translocation, and duplication
D) Only genetic changes occurring in regions of DNA that encode proteins are relevant to tumor progression
c
is cancer a new disease
Cancer is an ancient disease
The earliest mention of cancer comes from papyri of ancient Egyptians
but an understanding of cancer is recent
Histology
A branch of anatomy that studies the microscopic structure of tissues
A pathologist’s assessment of a tumor provides important information about
tissue of origin, diagnosis, prognosis, and treatment options
cancer stage
tumor size and location
How large is the tumor and has it spread to other tissues?
cancer grade
tumor morphology/structure and growth rate
How abnormal do the cells look and how fast are they growing?
why is determining the cell type of origin (and stage and grade) important when treating a cancer patient.
Cell type of origin reveals what normal cell the tumor came from—this informs drug response, molecular markers, and likely progression.
Stage (size and spread) helps guide treatment aggressiveness (e.g., localized vs metastatic).
Grade reflects how abnormal/dividing the cells are—high-grade tumors are usually more aggressive.
Different cancer types require different therapies, even if mutations are similar (e.g., lung vs colon cancer with same KRas mutation may not respond the same).
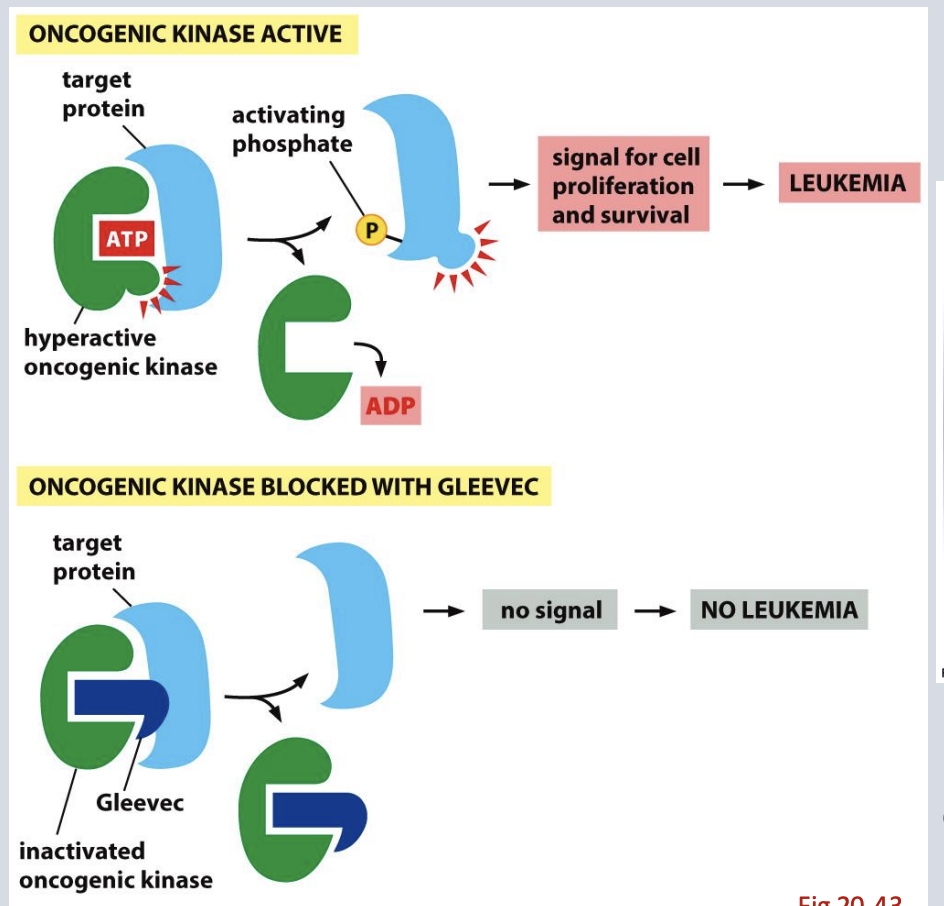
Explain what the Philadelphia chromosome is
Philadelphia chromosome = reciprocal translocation between Chr 9 & 22 → Bcr-Abl fusion.
Bcr-Abl = hyperactive tyrosine kinase → drives cell proliferation
why Philadelphia chromosome’s discovery supported the theory that cancer is a disease of altered hereditary material.
Proved cancer is a disease of altered chromosomes/DNA.
Describe what Bcr-Abl is and why it was a good drug target for development of Gleevec
Bcr -Abl is an in frame fusion of the coding regions of these genes caused by a reciprocal translocation between Chr 9 and Chr 22
The abl tyrosine kinase domain becomes hyperactive in Bcr -Ab
Gleevec targets Bcr-Abl kinase → effective especially in early-stage CML.
dominant vs recessive activities of oncogenes and tumor suppressors
Oncogenes = gain-of-function → dominant (1 mutated copy enough).
Tumor suppressors = loss-of-function → recessive (both copies must be lost).
Why does the landscape of mutations look so different in an oncogene vs tumor suppressor?
because oncogenes require specific gain of function mutations, often missense clustred in soecific regions, while tumor suppressors undergo widespread los of function mutation, including truncrating mutation to disrupt their function
mechanisms that could lead to activation of an oncogene
Oncogenes: point mutations (e.g., Ras), gene amplification (e.g., Myc), chromosomal rearrangements (e.g., Bcr-Abl).
mechanisms that could lead to suppression/loss of a tumor suppressor
Tumor suppressors: deletions, LOH, promoter methylation, nonsense mutations, dominant negative mutations (e.g., p53).
Explain why most people who inherit a mutation in Rb from a parent eventually develop retinoblastoma in both eyes
Once one copy of a tumor suppressor is mutated, there is an increased likelihood of loss of the remaining functional copy
Homologous recombination (homology directed repair) uses another chromosome as a template and is more active in mitotic cells. This type of repair can lead to loss-ofheterozygosity (LOH = “gene conversion”)
tumor microenvironment
the surrounding network of extracellular matrix, signaling molecules, and cell types such as immune cells, fibroblasts, blood vessels and resident normal tissue
- This is also referred to as the tumor stroma, which is a term for the supportive connective tissue and blood vessels in an epithelial organ (can make up to 90% of a tumor!)
tumor microenvironment’s role it has in tumor progression
Clonal evolution of cancer:
In the early stages of disease progression, genetic changes promoting cancer (ex. enhancing growth rate) arise and are inherited by daughter cells leading to clonal expansion as these cells outcompete their slower growing neighbors
Clonal expansions are further shaped by additional factors such as therapeutic intervention and metastasis
Heterogeneity
critical part of cancer genome evolution
Tumors are continually evolving
Driver mutations arise that cause expansion of unique subpopulations
Cancer can arise from between 2 and 20 key driver mutation
Genetic instability
promotes tumor progression
Chromosome abnormalities are a widespread feature of cancers
Extent of abnormalities correlates with clinical progression
what pathways are most frquently altered in cancer
Pathways involved in DNA damage response and repair
More cell division =
greater cancer risk
correalation between number of stem cell division and cancer risk
cyclins and cyclin-dependent kinases (Cdks)
control the different phases of the cell cycle
Rb
restricts entry into S phase
Hypophosphorylation of Rb prevents transcription of cyclin that initiates S phase
Hyperphosphorylation of Rb at the R point allows initiation of S phase (DNA replication)
Rb regulates the E2F transcription factor
Hypophosphorylated Rb binds E2F & prevents transcription
Hyperphosphorylated Rb (at the R point), releases E2F.
E2F then drives transcription of a cyclin the initiates S phase (DNA replication)
what does HDAC recruitment do?
they methylate → condense genes
Tumor progression requires loss of both copies of
Rb
MOST commonly mutated gene in cancer
p53 tumor suppressor
Humans who inherit a p53 mutation (Li-Fraumeni syndrome) are at high risk for many types of cancer
p53 in normal cells
Stresses activate p53 in normal cells
regulated primarily through degradation
regulation of p53
In non -stressed cells, the MDM2 ubiquitin ligase targets p53 for rapid degradation
Stress results in phosphorylation of p53 at the MDM2 binding site
Phosphorylated p53 is not degraded and can stimulate transcription of a variety of genes (Ex: for DNA repair, halting cell cycle, or promoting apoptosis)
Apoptosis is an important part of both development and tissue maintenance that becomes impaired in cancer cells
p53 function can be lost by
dominant negative missense mutations
p53 functions as a tetramer Even one mutant subunit in the tetramer makes the whole thing inactive
Mutations in the p53 enable cancer cells to
survive and proliferate despite stress and DNA damag
Hereditary cancers -
Inherited genetic variants have been linked to 5-10% of cancers in the U.S. -
Sporadic cancers
are caused by the accumulation of mutations that were not inherited from an individual’s parents
Carcinogen
An agent that causes cancer
Mutagen
An agent that causes genetic mutation
Chemotherapy and radiation therapy
preferentially target dividing cells and induce DNA damage