chapter 4: nomenclature and conformations of alkanes and cycloalkanes
0.0(0)
0.0(0)
Card Sorting
1/46
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
1
New cards
alkanes
C(n)H(2n+2)
sp3 / tetrahedral
sp3 / tetrahedral
2
New cards
cycloalkane
C(n)H(2n)
sp3/tetrahedral
simple ring, no extra bonds
sp3/tetrahedral
simple ring, no extra bonds
3
New cards
substituents
extensions of the main chain
- end in -yl
- end in -yl
4
New cards
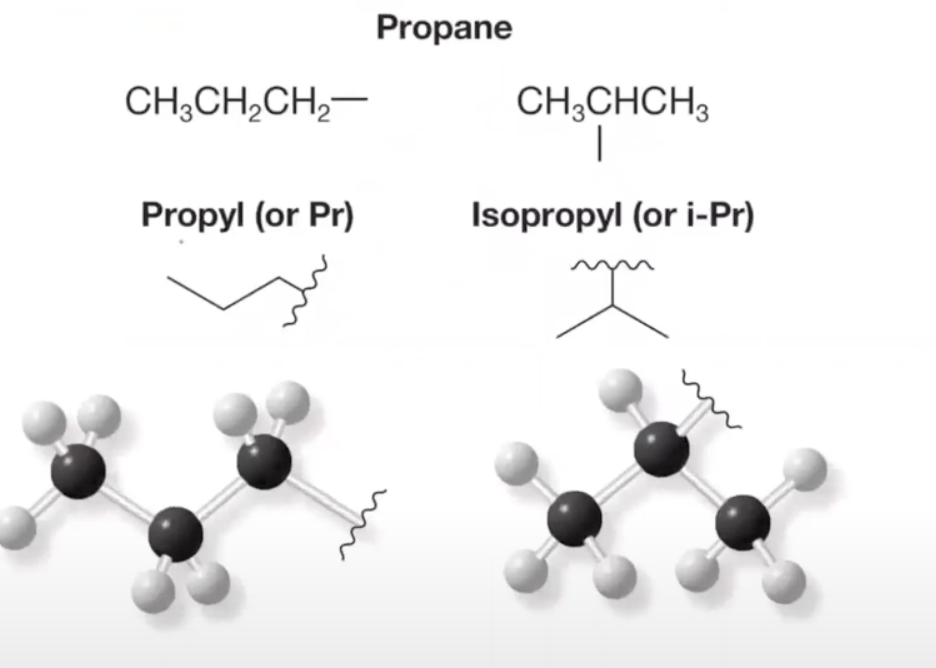
types of substituents: 3 carbons
- propYL (carbons in a chain)
- ISOpropYL (isomer/bunny ears)
- ISOpropYL (isomer/bunny ears)
5
New cards
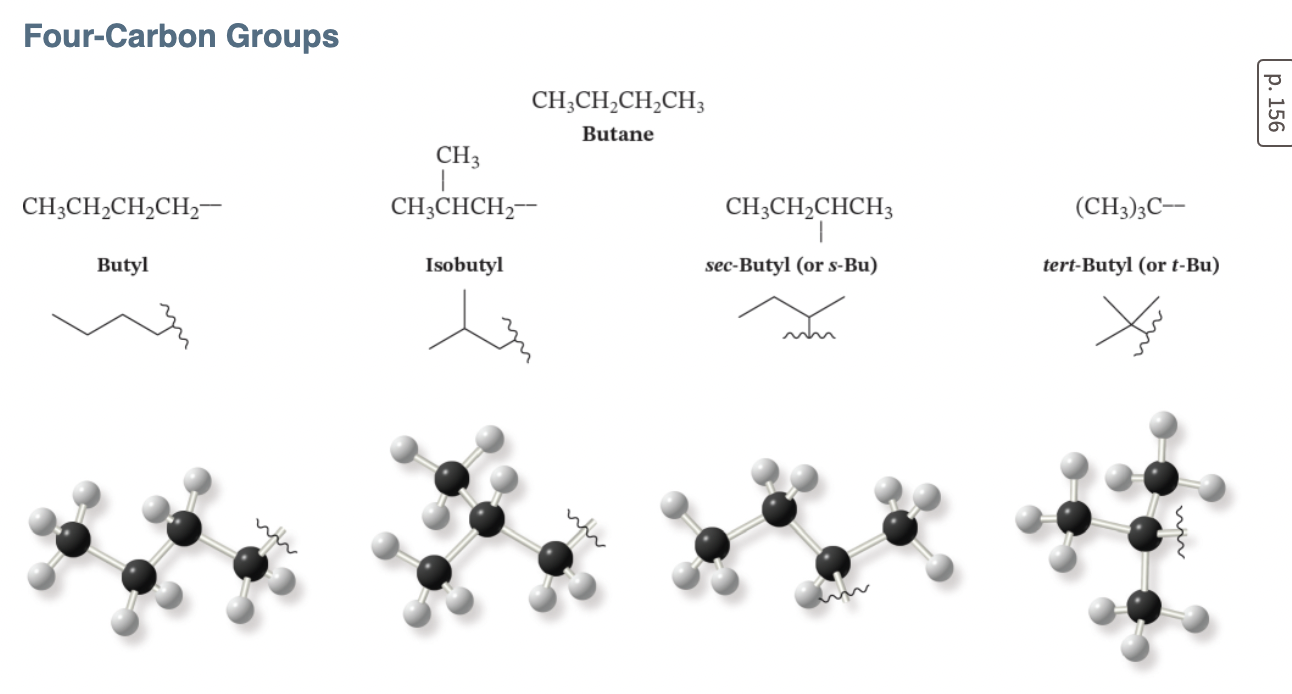
types of substituents: 4 carbons
butyl: straight
isobutyl: bunny ears at the end
sec-butyl: secondary carbon
tert-butyl: tertiary carbon
isobutyl: bunny ears at the end
sec-butyl: secondary carbon
tert-butyl: tertiary carbon
6
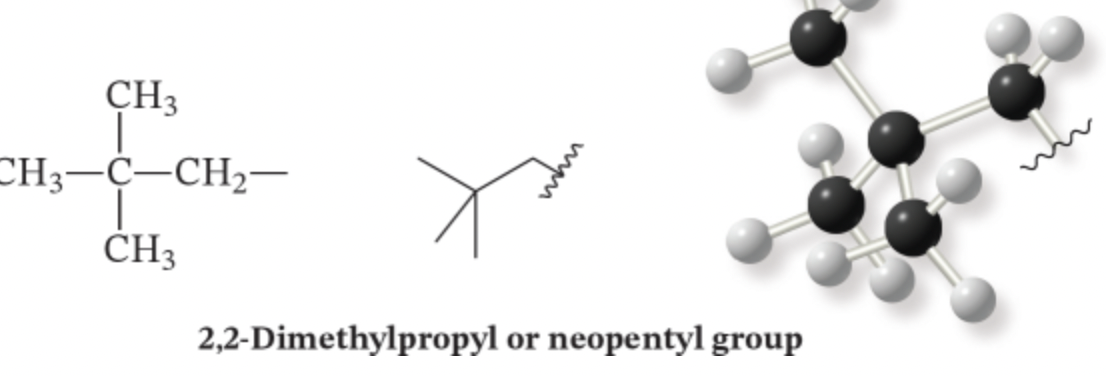
New cards
types of substituents: 5 carbons
neopentyl: butyl plus an extra carbon
7
New cards
parts of a systematic name
parent name: longest carbon chain
suffix: indicates which functional group is present
prefix: identity, location, and number of substituents
suffix: indicates which functional group is present
prefix: identity, location, and number of substituents
8
New cards
name branched chain alkanes
- find the longest chain
- give each substituent a name and number
- if one substituent, give it the lowest number on the chain. for 2 or more do the same and put alphabetically.
- when substituents are on the same chain use than number twice
- for identical substituents (ex 2 methyls) use prefixes like di, tri, tetra
when there are parent chains of equal length, choose the one with the greater number of substituents
list substituents in alpha order (not including prefixes or when when hyphenated)
when branches occur at an equal distance from either end of the chain, choose the lower set (ex: pick 2,3,5 over 2,4,5)
- give each substituent a name and number
- if one substituent, give it the lowest number on the chain. for 2 or more do the same and put alphabetically.
- when substituents are on the same chain use than number twice
- for identical substituents (ex 2 methyls) use prefixes like di, tri, tetra
when there are parent chains of equal length, choose the one with the greater number of substituents
list substituents in alpha order (not including prefixes or when when hyphenated)
when branches occur at an equal distance from either end of the chain, choose the lower set (ex: pick 2,3,5 over 2,4,5)
9
New cards
how to name branched alkyl groups
- find the longest chain
- give each substituent a name and number
- if one substituent, give it the lowest number on the chain. for 2 or more do the same and put alphabetically.
- when substituents are on the same chain use than number twice
- for identical substituents (ex 2 methyls) use prefixes like di, tri, tetra
when there are parent chains of equal length, choose the one with the greater number of substituents
list substituents in alpha order (not including prefixes or when when hyphenated)
when branches occur at an equal distance from either end of the chain, choose the lower set (ex: pick 2,3,5 over 2,4,5)
branched substituents are also named as their alkyl group names (ex iso, sec, tert)
- give each substituent a name and number
- if one substituent, give it the lowest number on the chain. for 2 or more do the same and put alphabetically.
- when substituents are on the same chain use than number twice
- for identical substituents (ex 2 methyls) use prefixes like di, tri, tetra
when there are parent chains of equal length, choose the one with the greater number of substituents
list substituents in alpha order (not including prefixes or when when hyphenated)
when branches occur at an equal distance from either end of the chain, choose the lower set (ex: pick 2,3,5 over 2,4,5)
branched substituents are also named as their alkyl group names (ex iso, sec, tert)
10
New cards
how to name cycloalkanes
cyclo- (number of carbons)-ane
ex: 5 = cyclopentane
- ring is the paren t hydrocarbon unless the substituent has more carbons than the ring [( when sub is larger: 1- cyclobutYLpentANE) (when parent is smaller: 1-methylcyclohexane)]
- when more than one substituent cite in alpha order, the first one will be numbered as 1
the exception to this is if you number them the other way around and produce lower set of numbers (ex: 2m, 4e, 1p >>> 1p, 3m, 4e7
ex: 5 = cyclopentane
- ring is the paren t hydrocarbon unless the substituent has more carbons than the ring [( when sub is larger: 1- cyclobutYLpentANE) (when parent is smaller: 1-methylcyclohexane)]
- when more than one substituent cite in alpha order, the first one will be numbered as 1
the exception to this is if you number them the other way around and produce lower set of numbers (ex: 2m, 4e, 1p >>> 1p, 3m, 4e7
11
New cards
cis-isomer
functional groups on same side
12
New cards
transisomer
functional groups on different sides
13
New cards
bicycloalkane
compound containing 2 fused or bridged rings
parts:
- bridgehead: common carbons to both rings
- bridge: each bond or chain of atoms connecting bridgehead
- bridged vs. fused: bridged rings have one or more carbons in the bridge (below); fused rings have zero carbons in the bridge
bridges are signified with numbers (descending)
- ex you can have a one carbon bridge, 2 carbon bridge etc
named "bicyclo[#.#.#]...ane"
parts:
- bridgehead: common carbons to both rings
- bridge: each bond or chain of atoms connecting bridgehead
- bridged vs. fused: bridged rings have one or more carbons in the bridge (below); fused rings have zero carbons in the bridge
bridges are signified with numbers (descending)
- ex you can have a one carbon bridge, 2 carbon bridge etc
named "bicyclo[#.#.#]...ane"
14
New cards
bicycloalkane nomenclature
- identify the parent name
- number the carbons (around the largest bridgehead, 2nd largest and smallest, give substituent the lowest number)
- identify the bridges, bracket them
- add substituents
- number the carbons (around the largest bridgehead, 2nd largest and smallest, give substituent the lowest number)
- identify the bridges, bracket them
- add substituents
15
New cards
alcohol nomenclature
end in -ol
- find largest carbon chain containing C bonded to OH group
- number carbon chain giving OH the lower number
- name other substituents
- add diol/triol when more than one hydroxy group
5-methyl-3-hexanol/5-methylhexa-3-ol
- find largest carbon chain containing C bonded to OH group
- number carbon chain giving OH the lower number
- name other substituents
- add diol/triol when more than one hydroxy group
5-methyl-3-hexanol/5-methylhexa-3-ol
16
New cards
cyclic alcohol nomenclature
cyclo- / -ol
- OH gets the number 1 position; it isn't required to number the 1 either
- can put number before -OH or in front
- OH gets the number 1 position; it isn't required to number the 1 either
- can put number before -OH or in front
17
New cards
alkenes nomenclature
- find largest carbon chain containing both the carbons on the double bond
- number carbon chain, give the double bond the lowest number
- add substituents, alphabetize
- when more than 1 double bond, change the "-ene" to "-adiene" or "atriene"
- double bonds are usually located between C1 and C2. the 1 is usually omitted. the ring is numbered to give the first sub the lowest number (if sub and DB are on same carbon , it is 1)
- number carbon chain, give the double bond the lowest number
- add substituents, alphabetize
- when more than 1 double bond, change the "-ene" to "-adiene" or "atriene"
- double bonds are usually located between C1 and C2. the 1 is usually omitted. the ring is numbered to give the first sub the lowest number (if sub and DB are on same carbon , it is 1)
18
New cards
alkenols
compounds containing double bonds and hydroxys
19
New cards
cycloalkenes
20
New cards
allyl group
C=C-C-C
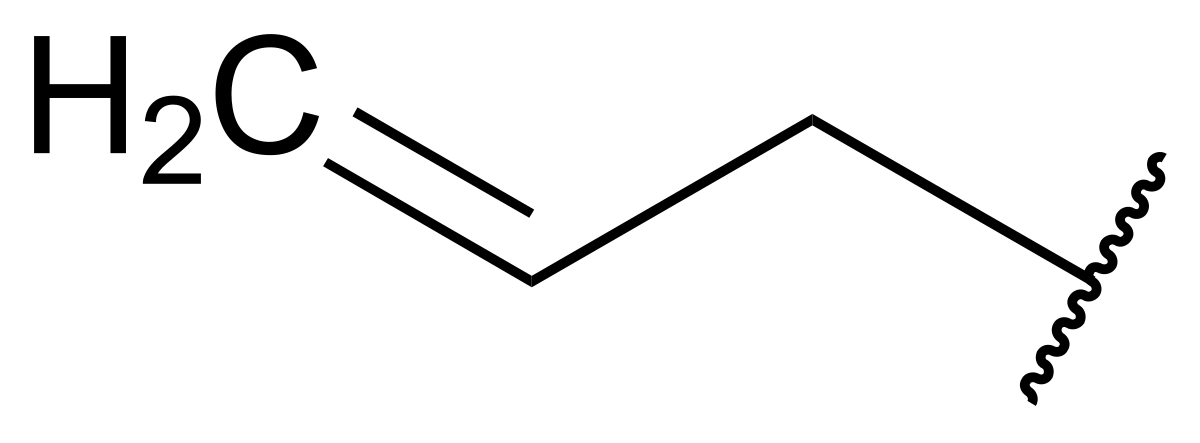
21
New cards
vinyl group
C=C-C
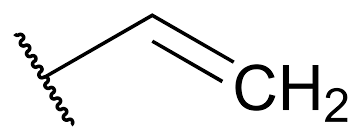
22
New cards
naming alkynes
-yne ending
-choose the longest parent chain containing both atoms in the triple bond, giving the triple bond the lowest number
- zdd substituents, alphabetize
- 2 triple bonds: diyene
- 3 triple bonds triyene
- "enyne": double bond and triple bond
-choose the longest parent chain containing both atoms in the triple bond, giving the triple bond the lowest number
- zdd substituents, alphabetize
- 2 triple bonds: diyene
- 3 triple bonds triyene
- "enyne": double bond and triple bond
23
New cards
alkyl halide nomenclature
always named as a substituent
Cl - chloro, F,-fluoro Br-bromo, I-iodo
- find the longest parent chain
- give the lowest number
alphabetize
Cl - chloro, F,-fluoro Br-bromo, I-iodo
- find the longest parent chain
- give the lowest number
alphabetize
24
New cards
conformations
different arrangements of atoms that are interconverted by rotation about single bonds
25
New cards
dihedral angle
angle separating a bond on one atom from a bond on an adjacent atom
- 0 dihedral angle- "eclipsed conformation" when the hydrogen groups on adjacent atoms are lined up on each other
- 60 dihedral angle- "staggered conformation" the furthest that the hydrogens on adjacent atoms can be spread apart. these have the lowest energy conformation
- 0 dihedral angle- "eclipsed conformation" when the hydrogen groups on adjacent atoms are lined up on each other
- 60 dihedral angle- "staggered conformation" the furthest that the hydrogens on adjacent atoms can be spread apart. these have the lowest energy conformation
26
New cards
sigma bonds ------ rotate, pi bonds ------ rotate
can; cant
27
New cards
newman projections
convention for drawing end-on representation of conformations
28
New cards
how to draw newman projections
- look directly down at the C-C bing (end on) and draw a circle with a dot in the center to represent the carbons of the bond
- draw the bonds on the front C as 3 lines meeting the center. next draw the bonds on the back c as 3 lines coning out of the edge of the circle (staggered first)
- add the atoms on each bond
- bods can be anything ex: hydrogens, methyl groups
- draw the bonds on the front C as 3 lines meeting the center. next draw the bonds on the back c as 3 lines coning out of the edge of the circle (staggered first)
- add the atoms on each bond
- bods can be anything ex: hydrogens, methyl groups
29
New cards
anti conformation
(newman conformation) a staggered conformation with 2 larger groups 180 degrees from each other
lowest energy
lowest energy
30
New cards
gauche conformation
(newman conformation) a staggered conformation with 2 larger groups 60 degrees from each other
31
New cards
eclipsed conformation
(newman conformation) - 0 dihedral angle; when the hydrogen groups on adjacent atoms are lined up on each other
highest energy when large groups right next to each other
highest energy when large groups right next to each other
32
New cards
staggered conformation
- 60 dihedral angle; the furthest that the hydrogens on adjacent atoms can be spread apart. these have the lowest energy conformation
lowest energy when large groups 180 degrees away from each other (anti)
lowest energy when large groups 180 degrees away from each other (anti)
33
New cards
steric strain
an increase in energy resulting when non bonded atoms are forced too close to one another (basically- when they pass each other as they rotate, the energy increases)
the relative energies of the individual staggered conformations depends on the amount of steric strain (ex more steric strain when 2 methyl groups come in contact rather than when 2 hydrogens come in contact)
gauche conformations are higher energy than anti conformations because of steric strain
the relative energies of the individual staggered conformations depends on the amount of steric strain (ex more steric strain when 2 methyl groups come in contact rather than when 2 hydrogens come in contact)
gauche conformations are higher energy than anti conformations because of steric strain
34
New cards
barrier to rotation
the energy difference between the lowest and highest energy conformations due to steric strain
35
New cards
what happens when you add more substituents to your newman conformations?
the energy will be greater
36
New cards
zigzag skeletal structures
the lowest energy conformations in alkenes are found in these structures because all groups are staggered and the large groups are anti
37
New cards
ring strain in cycloalkanes
is a combination of the following
- torsional strain: forced eclipsed conformation
- angle strain: an increase in energy when bond angles deviate from 109.5 where it is ideal for sp3 hybridized carbons
- torsional strain: forced eclipsed conformation
- angle strain: an increase in energy when bond angles deviate from 109.5 where it is ideal for sp3 hybridized carbons
38
New cards
what is they reality of the structure of cycloalkanes?
they pucker/distort their shapes in order to alleviate ring strain
ex- cyclohexanes will pucker to go from 120 degrees apart to 109.5 degrees
ex- cyclohexanes will pucker to go from 120 degrees apart to 109.5 degrees
39
New cards
chair conformation
most stable conformation of cyclohexene- eliminated angle strain (all C-C bonds 109.5 degrees) and torsional strain
each C in cyclohexenes have 2 different kinds of bonds to substituents (h or other groups)
- axial bonds: point up and down
- equatorial: point out
each C in cyclohexenes have 2 different kinds of bonds to substituents (h or other groups)
- axial bonds: point up and down
- equatorial: point out
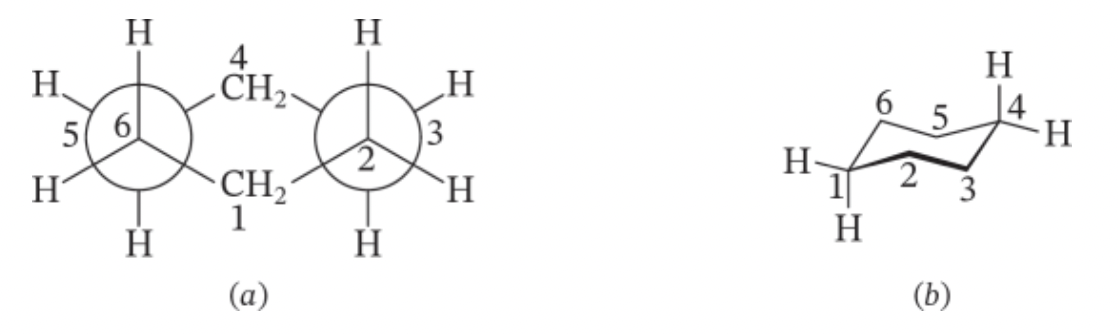
40
New cards
cyclohexene boat confromation
one side of the chair conformation will flip upwards
- flagpole positions: at the tips of the boat pointing inward, too much overlap causing steric strain
- not as stable because eclipsing
- flagpole positions: at the tips of the boat pointing inward, too much overlap causing steric strain
- not as stable because eclipsing
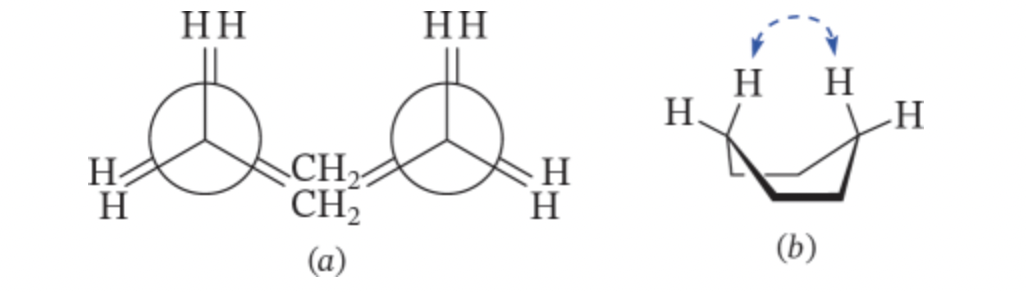
41
New cards
homologous series
when looking ar physical properties, a series of compounds in which each member differs from the next by a constant unit is called this
members of these series are called homologues
members of these series are called homologues
42
New cards
boiling points of alkanes and cycloalkanes
branching an alkane lowers the boiling point while in unbranched alkanes, when the molecular weight increases the size and molecular surface area increase thus dispersion forces increase and more energy is required to separate molecules (higher bp)
in addition to this, cycloalkanes will have higher boiling points than alkanes
in addition to this, cycloalkanes will have higher boiling points than alkanes
43
New cards
melting points of alkanes and cycloalkanes
in unbranched alkanes, you cannot see the melting point as smoothly as you would on the plot for boiling point. however, is you separate the curve with even and odd amounts of carbons, there is a smooth increase in the melting point. this is because when there are even amounts of carbons, the atoms pack more evenly in a crystalline state. thus attractive forces are greater and melting points are higher
in addition to this, cycloalkanes will have higher melting points than the open chains
in addition to this, cycloalkanes will have higher melting points than the open chains
44
New cards
mono-substituted cyclohexanes
to minimize the energy caused by steric strain, the ring can flip to put bulkier substituents in equatorial positions
molecules are changing conformations to minimize 1,3,-diaxial interactions of bulky substituents
- big substituents will try to be in equatorial position**
molecules are changing conformations to minimize 1,3,-diaxial interactions of bulky substituents
- big substituents will try to be in equatorial position**
45
New cards
degree of unsaturation / index of hydrogen deficiency
is the number of pi bonds and cyclic structures in a molecule
- note that a double bond has 1 pi bond while triple bonds have 2
- unsaturated hydrocarbons have less than the maximum number of hydrogen atoms per carbon (2n+2) [ alkenes/alkynes]
- note that a double bond has 1 pi bond while triple bonds have 2
- unsaturated hydrocarbons have less than the maximum number of hydrogen atoms per carbon (2n+2) [ alkenes/alkynes]
46
New cards
calculating degree of unsaturation
follow the general formula of a hydrocarbon (C[n]H[2n+2-2x])
2n+2 -2x; x = degree of saturation
- n = number of carbons
- when heteroatoms are present: ignore oxygen, add 1 for each halogen (F, Cl, Br, I) to the number of H's; subtract 1 from H for each N present
2n+2 -2x; x = degree of saturation
- n = number of carbons
- when heteroatoms are present: ignore oxygen, add 1 for each halogen (F, Cl, Br, I) to the number of H's; subtract 1 from H for each N present
47
New cards
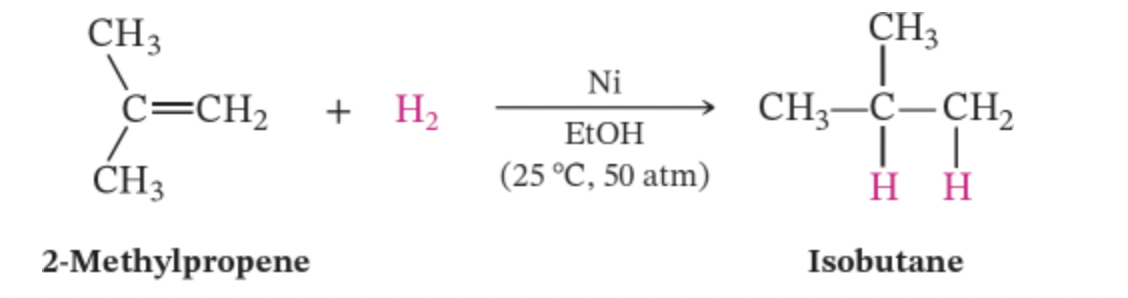
hydrogenation
a reaction in which a hydrogen adds to a double or triple bond. often completed trhough the use of a metal catalyst like Platinum Pt, palladium Pl, or nickel Ni.
the alkene and alkynes will react with hydrogen in order to produce alkanes
the alkene and alkynes will react with hydrogen in order to produce alkanes