6 - Raman and FTIR Spectroscopy
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is spectroscopy?
Spectroscopy:
Investigation and measurement of spectra produced when matter interacts with or emits electromagnetic radiation.
What is spectrometry?
Spectrometry:
Analysis of measured intensities in various parts of a spectrum
Electromagnetic radiation can interact with matter in the following ways :
No interaction
Elastic scattering (no change in energy)
Excitation of the sample (some band of energy is absorbed)
Inelastic scattering (Energy exchange)
What is vibrational spectroscopy?
Molecules vibrate: Imagine atoms (balls) are joined by chemical bonds (springs).
Vibrational modes for a molecule are a function of the orientation of its atoms and bonds, the atomic mass of the atoms, and other factors.
Each vibrational mode is initiated by a specific frequency. These modes are quantized much like atomic energy levels.
What is Raman Spectroscopy?
A spectroscopic technique typically used to determine vibrational modes of molecules for identification and analysis.
Why use Raman Spectroscopy?
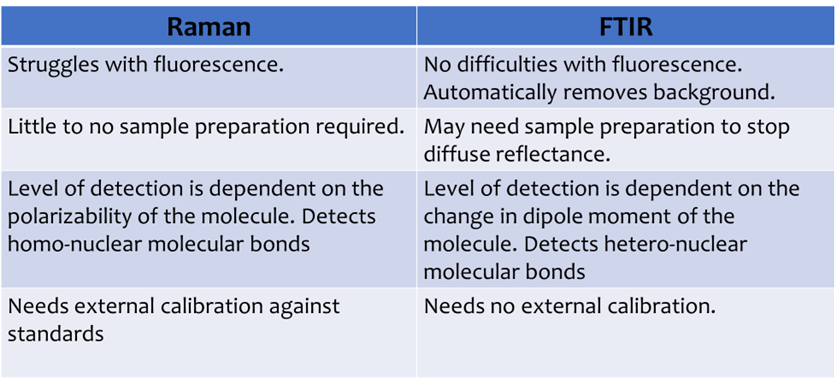
1) A ‘non-destructive’ technique with little to no sample preparation. Can analyse fluids through glass containers.
2) Quick and cheap identification of minerals and other compounds.
3) Good for studying phase transformation in polymorphs.
4) Good for studying organics, including changes with temperature.
As an oversimplification: Raman is used to study the bonds between atoms. It does not provide information on elemental composition.
What is the Raman effect?
• Incident light is shone from a monochromatic laser source.
• Most of this light is (elastically) scattered at the same wavelength – Rayleigh Scatter.
• A small amount of the laser light interacts with the molecular bond vibrations and scatters with a change in energy (+/-) – Raman Scatter.
Technically, any laser wavelength can be used to generate a Raman spectrum, provided sufficient laser intensity is delivered to the sample. 532 nm is best for inorganic (geological) samples.
The ‘Raman effect’ occurs when the incident laser causes a momentary polarization of a molecule, transferring energy that is immediately re-emitted. If the molecule gains energy, then the scattered photon loses energy, causing the photon wavelength to increase.
The energy difference between the incident laser and the emitted Raman photon is equal to that between the initial and final rotational, and/or vibration states of the molecule (rovibronic).
E = hυ
Energy = Planck’s constant × Frequency
υ = c/λ
Frequency = speed of light / wavelength
What instrumentation is used for Raman?
A grating splits scattered light and projects it onto a CCD which is used to count the intensity at specified ‘energy’ intervals.
Reads onto a computer.
How is sample preparation for Raman spectroscopy?
Raman Spectroscopy needs very little sample preparation!
For geological samples you can use an uneven surface, polished rock, power, or create a thin section. Water is a very weak Raman scatterer so Raman can be performed directly on the fluid sample. Glass or polymer containers also give little to no signal.
What standards are used for Raman spectroscopy?
Raman instrumentation needs to be calibrated. Sensitive equipment needs to be adjusted so that peaks for a known material are always the same. This ensures data from research can be compared to each other.
How is data acquisition for Raman spectroscopy done?
Collecting a single spectra shows the scattering of compounds present under the width of the laser spot directed at a sample. This spectra might be the scattering from one compound or multiple, meaning peaks may be superposed one on top of each other.
Collecting lots of spectra together, running the laser over the sample (like a raster) mean we can ‘image’ a sample. This is called hyperspectral mapping. It is good for the in-situ study of context within samples.
What are the possible sources of error for Raman?
When studying Raman spectra, we need to consider possible sources of error or spectral artifacts that we need to remove/correct for:
1) Background reduction / removal
• Fluorescence
• Noise
2) Cosmic ray reduction / removal
What are the pros and cons of Raman?
What is FTIR?
Fourier Transform Infra-Red (FTIR) Spectrometry
Why use FTIR?
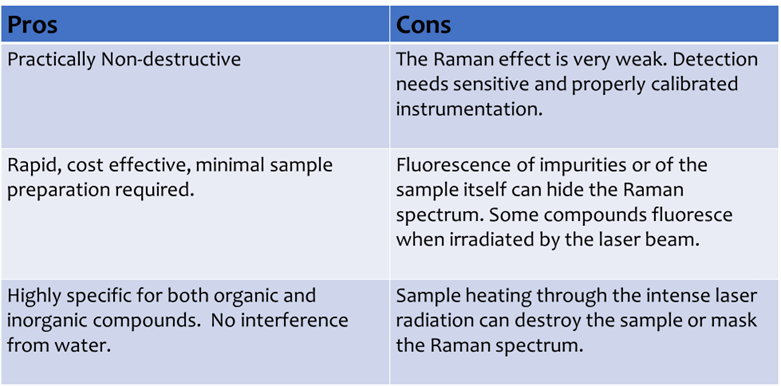
1) Can quickly and definitively identify compounds.
2) Organic and inorganic compounds can be identified.
3) Highly sensitive and needs no external calibration.
4) Ideal for small amounts of liquid samples or powdered solids.
IR light has a wavenumber range spanning ~12,800 cm-1 to 10 cm-1
FTIR is ‘mid range’ and normally looks at 4,000 cm-1 to 400 cm-1
What is IR spectroscopy?
Conventional infrared spectrometers have two beams of radiation, one passing through the sample, the other passing through a reference. As a particular frequency is absorbed by the sample, less radiation is transmitted. The detector compares the intensity passing through the sample with the intensity passing through the reference (which might just be air).
A monochromator is used to select radiation of only one frequency at a time.
Unlike Raman spectroscopy, which measures relative frequencies at which a sample scatters radiation, IR spectroscopy measures absolute frequencies at which a sample absorbs radiation.
IR / FTIR:
• Sensitive to heteronuclear functional group vibrations and polar bonds, such as OH.
• Won’t detect homonuclear molecular bonds such as C-C or N-N.
IR spectroscopy detects the change in dipole moment. A dipole is the separation of positive and negative charges that will change during the vibrational motion of a molecule.
What is the Michelson interferometer?
Transmitted light and reflected light strike a moveable mirror and reflect back, recombining at the beam splitter. By moving the mirror and causing interference of the beam, spectral information for multiple frequencies is collected.
• Extra distance travelled by light striking moveable mirror = optical path difference (OPD).
• If OPD is multiple of λ, constructive interference occurs, resulting in maximum intensity signal.
• When OPD is half of λ, destructive interference occurs, resulting in minimum intensity signal.
What is the Fourier transformation?
A spectrum and its interferogram are related via a “Fourier transformation”.
Unlike IR, FTIR is constructed from an ‘interferogram’ as the raw signal. The transmittance intensity is a function of the mirror inside, not wavelength.
By completing a Fourier Transformation, we can decompose this into a function of wavelength.
A spectrum and its interferogram are related via a “Fourier transformation”, which is a mathematical transform that decomposes functions into frequency components.
But by completing a Fourier Transformation, we can decompose this into a function of wavelength.
What are the different modes of analysis for FTIR?
Samples preparation depends on the mode of analysis:
Transmission
Specular Reflectance
Attenuated Total Reflectance (ATR)
Diffuse Reflectance
What are the pros and cons of FTIR?
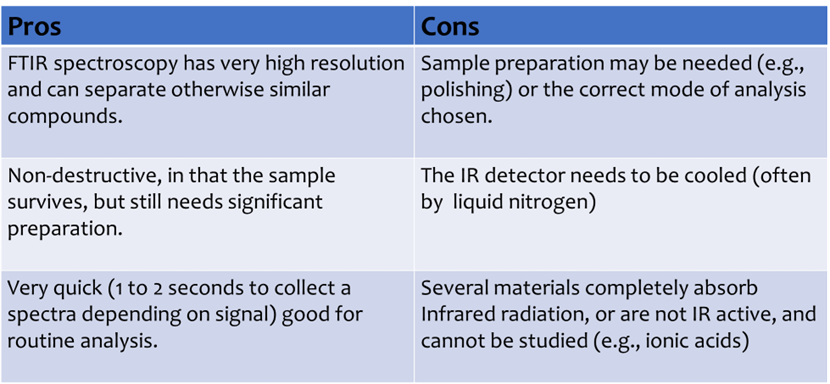
Compare Raman and FTIR?
Both FTIR and Raman spectroscopy can be used to detect (qualitatively) organic and inorganic substances. More sophisticated analyses can study the physical properties of a substance and how it might change with pressure, temperature, time, etc.