ORGO FINAL REAGENTS
1/24
Earn XP
Description and Tags
ORGO321: Organic Chemistry 1 - Final Reagents *mostly just did the weird ones you have to memorize*
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Hg(OAc)2 / NaBH4
alkene (chain) —> OH group
mCPBA
epoxide
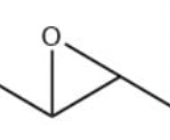
a) O3
b) DMS
alkene (chain) —> splits into two with O= ends
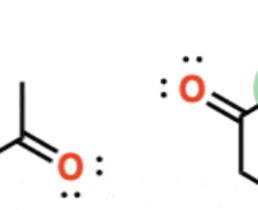
HBr, light, ROOR
alkene (chain) —> adds Br
2: H2, Pd
alkene (chain) —> alkane
a) BH3
b) HOO-
alkene (ring) —> OH group
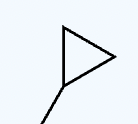
CH2N2
carbon triangle
a) OsO4
b) NaOH, H2O
alkene (ring) —> 2 OH groups
excess HBr + alkyne
2 Br
excess Br2 + alkyne
4 Br
HgSO4a H2SO4, H2O
alkyne —> ketone
a) HB(Sia)2
b) HOO-
alkyne —> aldehyde
NaNH2
deprotonates alkynes
NaH
deprotonates alcohols
H2, Lindlar’s
alkyne —> Z alkene
Li, NH3(l)
alkyne —> E alene
3: H2, Pd
alkyne —> alkane
a) LiAlH4
b) H+
ketone/aldehyde —> OH group
OH, H2O
ketone —> 2 OH groups
OCH3-, HOCH3
ketone —> OH group and OCH3
a) gringard (chain to MgBr)
b) H+
ketone/aldehyde —> chain and OH group
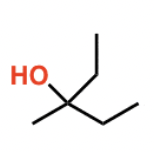
LDA
deprotonates alkanes: alkane w/ LG —> alkene
excess NaNH2 heat
alkane w/ LG —> alkyne
:alkyne
alkane w/ LG —> adds alkyne
conc. H2SO4 heat
alcohol —> alkene