Chemistry Exam 4
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
addition of small amounts of reagent to a solution until the equivalence point has been reached
Titration
the number of moles of acid and base are stoichiometrically equal
Equivalence Point
a solution of unknown concentration
Titrant
may be added to determine when the equivalence point has been reached. A chemical that changes color with pH.
Indicator
the point of pH change where the indicator changes color
Endpoint
endpoint ; equivalence point
Indicators are selected so that the ______ coincides with the predicted ___________
equivalence point of the titration
Inflection point
pH of the salt solution
The pH of the equivalence point depends on the ________________
Carbonic acid and bicarbonate ; H₂CO₃→CO₂+H₂O
OH⁻+H₂CO₃→HCO₃⁻+H₂O
An important buffer in the blood is the mixture of _________________________________. Write the two reactions that prove how the increase in [H+] or [OH-] in blood is counteracted by the buffer components in blood.
A. 2.78
B. 9.37
C. 4.74
Calculate the pH of the following solutions, Ka acetic acid =1.8 x 10-5 :
a. 0.15 M acetic acid solution
b. 0.15 M sodium acetate solution
c. a solution containing 0.15 M acetic acid and 0.15 M sodium acetate
1.75
A solution containing NH3(aq) and NH4Cl(aq) has a pH of 9.5. What is the [NH3]/[NH4 + ] ratio in this solution? (For ammonia, Kb = 1.8 x 10–5 .)
A. 4.52
B. 4.52
C. Diluting a buffer does not change its pH, because the acid/base ratio stays the same.
A 100 mL buffer solution containing 0.25M CH3COOH and 0.15M CH3COONa is placed into a volumetric flask and diluted to 250 mL with water.
a) calculate the pH of the initial solution (when the volume was 100 mL)
b) calculate the pH of the final solution, after dilution (when the volume is 250mL)
c) what can you conclude?
A. 4.93
B. 5.07
You have 500.0 mL of a buffer solution containing 0.20 M acetic acid (CH3COOH) and 0.30 M sodium acetate (CH3COONa).
a) What is the pH of this buffer?
b) What will the pH of this solution be after the addition of 20.0 mL of 1.00 M NaOH solution? [Ka = 1.8 x 10–5 ]
21.0 mL
What volume of 1.00 M sodium hydroxide should be added to 250 mL of 0.100 M HCOOH to obtain a solution with a pH of 4.50? [Ka(HCOOH) = 1.7 x 10–4 ]
8.92
Calculate the pH at the equivalence point for the titration of 100 mL 0.25 M CH3COOH with 100 mL of 0.25 M NaOH. (For CH3COOH, Ka= 1.8 x 10–5 )
A. 100.0mL
B. 1.70
A 100.0 mL sample of 0.10 M NH3 is titrated with 0.10 M HNO3.
A) What volume of HNO3 is needed to reach the equivalence point in this titration?
B) Determine the pH of the solution after the addition of 150.0 mL of HNO3.
4.75
A mixture made from 10 mL of 1.0 M HCl and 20 mL of 1.0 M CH3COONa would be classified as a buffer solution. Prove that this solution will work as a buffer. Calculate the pH of this solution. CH3COOH, Ka= 1.8 x 10–5
29.5mL
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 0.0990M HBr to the equivalence point?
4.20
What is the pH of the resulting solution if 45.00 mL of 0.10 M acetic acid is added to 10.00 mL of 0.10 M NaOH? Assume that the volumes of the solutions are additive. Ka = 1.8 × 10-5 for CH3COOH.
1.07 x 10–8
The solubility of lead(II) iodide is 0.064 g/100 mL at 20oC. What is the solubility product for lead(II) iodide?
1.1 x 10–5 mol/L
The solubility product for barium sulfate is 1.1 x 10–10. Calculate the molar solubility of barium sulfate.
3.0 x 10–2 M
Calculate the silver ion concentration in a saturated solution of silver(I) sulfate, knowing Ksp = 1.4 x 10–5
9.22 × 10-9 M
Determine the molar solubility of AgI in pure water. Ksp (AgI) = 8.51 × 10-17
A. 0.0162 M
B. 0.0017 M
a) Lead (II) chloride is dissolved in water to form a saturated solution. Calculate the molar concentration of Pb2+, [Pb2+], in this solution, knowing that Ksp = 1.7 x 10-5
b) Calculate the molar concentration of Pb2+, [Pb2+], if PbCl2 would be dissolved in 0.10 M NaCl solution, knowing that Ksp = 1.7 x 10-5 . Is this [Pb2+] larger or smaller that in a)?
0.55M
Determine the total concentration of Cl- , [Cl- ], in a solution that contains 0.10 M NaCl, 0.15 M KCl, 0.15 M CaCl2.
4.11 x 10-7 M
Gout is a condition that results from the precipitation of sodium urate crystals in tendons, cartilage and ligaments. If the [Na+ ] in blood is 0.140 M and the Ksp for sodium urate is 5.76 x 10 -8 , what is the minimum concentration of urate that results in the precipitation of sodium urate?
A. 1.0
B. 0.10 M
C. 50mL
D. 10mL
Assume HCl (in the beaker) was titrated with NaOH (in the burette) and they had the same concentration.
a) Read the pH of the solution in the beaker before any base was added to it; pH _____
b) HCl strong acid → [HCl] start = ___ M
c) Estimate the volume of HCl that was in the beaker at the start of titration; vol HCl = _______ mL
d) estimate from the graph the volume of NaOH that was added in 5 excess (beyond the equivalence point) during this titration vol NaOH excess = ___ mL
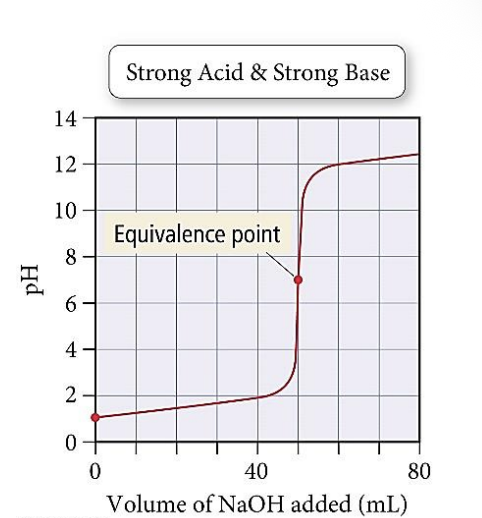
A. 7
B. 11.96
A. What is the pH at the equivalence point in this titration?
B. What is the pH of a solution after mixing 50.0 mL of 0.1 M HCl and 60.0 mL 0.1 M NaOH?
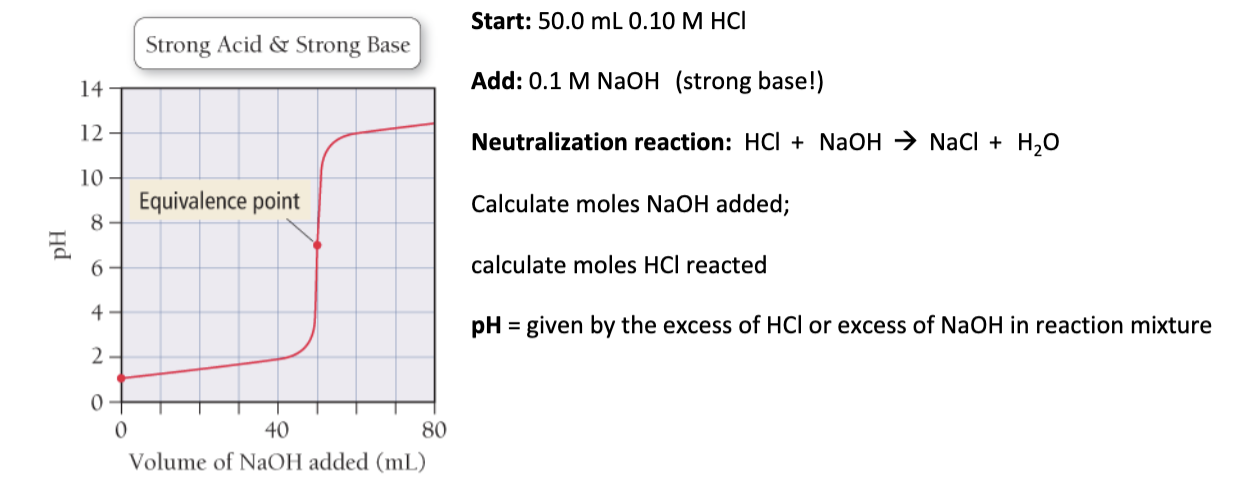
A. 2.38
B. 3.93
C. 25.0mL
D. 8.22
E. 12.22
A. What is the pH of a 0.1 M HCOOH solution?
B. What is the pH of a solution after mixing 25.0 mL 0.1 M HCOOH with 15.0 mL 0.1 M NaOH? HCOOH + NaOH → HCOO Na + H-OH
C. How many mL of a 0.1 M NaOH solution is needed to reach the equivalence point when starting with 25.0 mL 0.1 M HCOOH?
D. Calculate pH at the equivalence point.
E. Calculate the pH of a solution after mixing 25.0 mL 0.1 M HCOOH with 35.0 mL 0.1 M NaOH.
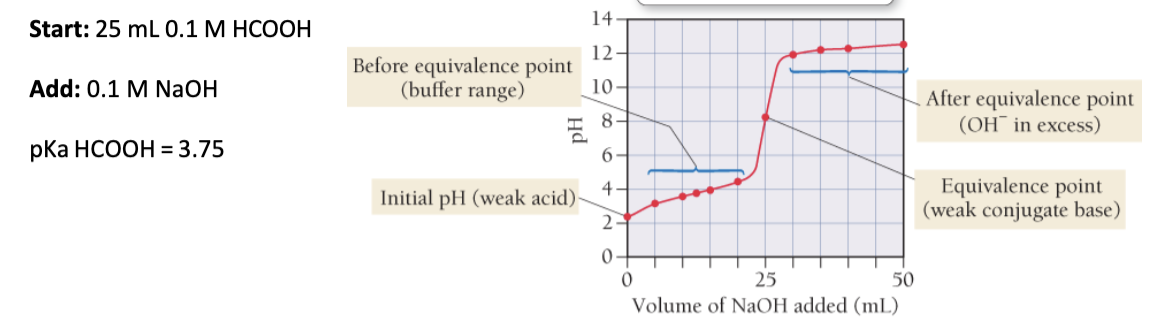
glycolic acid and oxalic acid
Antifreeze is metabolized in the liver to ________ and _________
drop
In the bloodstream, glycolic acid overwhelms the buffering ability of the HCO3– in the blood, causing the blood pH to ______
solutions that resist changes in pH when an acid or base is added
Buffer solutions
weak acid and its conjugate base
A buffer solution must contain significant amounts of both a _______ ______ and its ______ ______
The added base will be neutralized by the weak acid component in the buffer
What happens to a buffer solution when a small amount of strong base is added?
The added acid will be neutralized by the conjugate base component in the buffer
What happens to a buffer solution when a small amount of strong acid is added?
moderate
A good buffer should be able to neutralize ________ amounts of added acid or bas
pH
There is a limit to how much buffer can be added before the _______ changes significantly
the pH range at which the buffer can be effective
Buffering range
amount of acid or base that can be added to a buffer without causing a large change in the pH of the buffer
Buffering capacity
low ; insoluble
Many compounds have such _____ solubility in water that they are classified as ______
the equilibrium constant for the dissociation of a solid salt into its aqueous ions
Solubility-product constant (Ksp)
the amount of solute that will dissolve in a given amount of solution at a particular temperature
Solubility
the number of moles of solute that will dissolve in a liter of solution
Molar solubility
lower ; higher
For insoluble ionic compounds that contain anions of weak acids, the ______ the pH, the ____ the solubility
above ; precipitate
If Q > Ksp, the solution would be ______ saturation. The salt above saturation will ________
solutions with Q > Ksp will not precipitate unless disturbed
Supersaturated solutions
different
A successful reagent can precipitate with more than one of the cations, as long as their Ksp values are significantly _______
ions that form by combining a cation with several anions or neutral molecules
Complex ions
attached ions or molecules
Ligands
the reaction between an ion and ligands to form a complex ion is called a complex ion formation
Complex ion formation
the equilibrium constant for the formation reaction
Formation constant, Kf
reaction between an acid and a base → salt + water
Neutralization reaction
Spontaneous
Is rusting of iron spontaneous or non-spontaneous?
Non-spontaneous
Is electrolysis spontaneous or non-spontaneous?
A. -
B. -
C. +
D. -
Predict the sign of ΔS (positive or negative) for these reactions without using a table for the individual entropy values:
A. CH3OH(l) → CH3OH(s)
B. N2(g) + 3 H2(g) → 2 NH3(g)
C. CH4(g) + H2O(g) → CO(g) + 3 H2(g)
D. Na2CO3(s) + H2O(g) + CO2(g) → 2 NaHCO3(s)
-121.2 J/K
Calculate the change in entropy when 20.0 g of H2O condenses from a gas to a liquid at the normal boiling point of water, 100.0 °C. ΔHvap = 40.7 kJ/mol
Mg(s) < Pb(s) < CH₄(g) < C₂H₆(g)
Place the following in order of increasing entropy at 298 K (individual S° values not needed).
C2H6(g), Pb(s), Mg(s), CH4(g)
Cl₂(g)
Predict which one of the following has the highest standard molar entropy, S°, at 25°C? H2(g); O2(g); N2(g); Cl2(g)
-112.3 J/K
Calculate ΔS°rxn for the following reaction. The S° for each species is shown below the reaction. C2H2(g) + H2(g) → C2H4(g)
S°(J/mol∙K) 200.9 130.7 219.3
+178.8 J/K
Calculate ΔS°rxn for the following reaction. The S° for each species is shown below the reaction. 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
S°(J/mol∙K) 192.8 205.2 210.8 188.8
-990.3 kJ; spontaneous at 25C
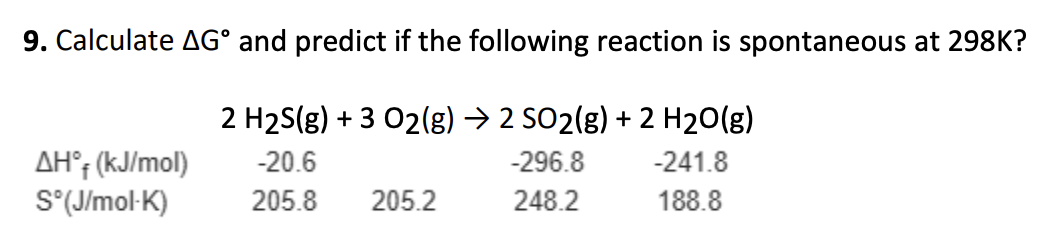
A. +53.3 kJ
B. 673 K
Consider the reaction: CCl4 (g) → C (s, graphite) + 2 Cl2 (g) ΔH = +95.7 kJ; ΔS = +142.2 J/K
a. Calculate ΔG° at 25 °C and determine whether the reaction is spontaneous
b. At what temperature will the reaction become spontaneous?
632 K
Above what temperature does the following reaction become nonspontaneous?
FeO(s) + CO(g) → CO2(g) + Fe(s)
ΔH = -11.0 kJ;
ΔS = -17.4 J/K
+137.2 kJ
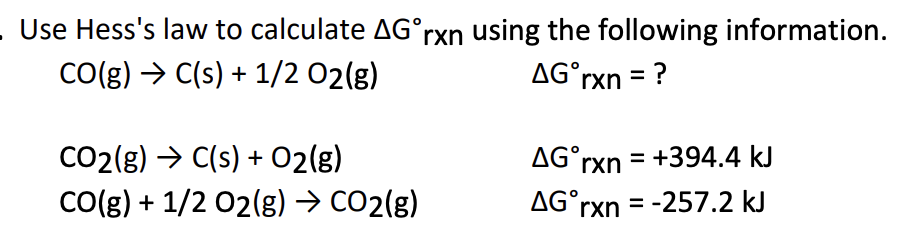
A) K=1.05×10−23K = 1.05 \times 10^{-23}K=1.05×10−23
B) K=4.93×1031K = 4.93 \times 10^{31}K=4.93×1031
C) K=7.17×10−58K = 7.17 \times 10^{-58}K=7.17×10−58
D) K=8.09×104K = 8.09 \times 10^{4}K=8.09×104
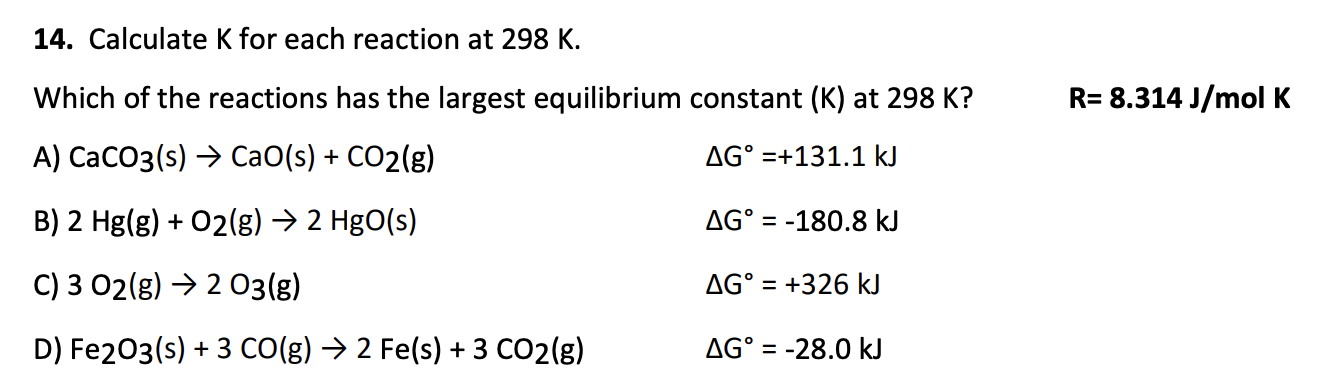
ΔG° = +51 kJ
K = 1.15 × 10-9
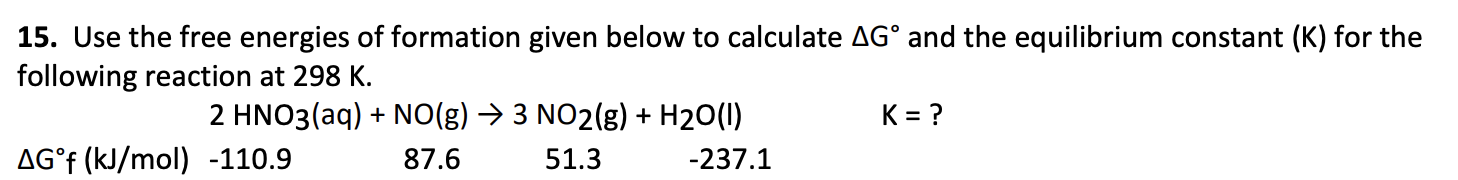
ΔG° = +28.19 kJ
K =2.08 × 10-3

54 kJ/mol
The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) ⇌ 2NH3(g) is
Kp = 9.4 x 10–5 . Calculate the value of ΔG° for the reaction under these conditions.
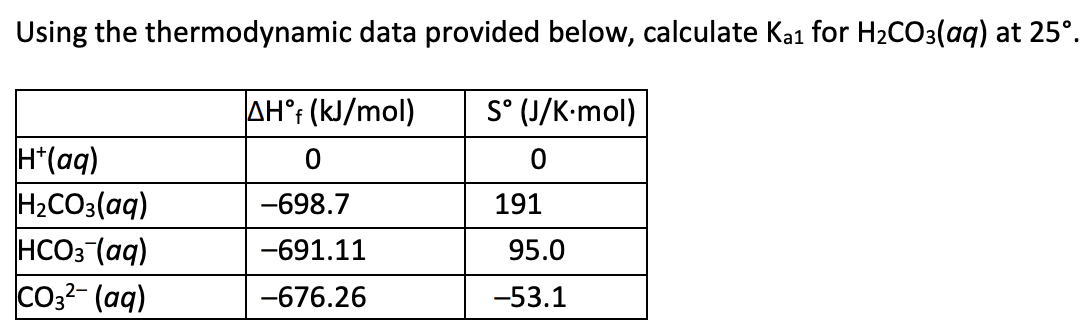
4.5 × 10^-7
thermal equilibrium; when two objects are separately in thermodynamic equilibrium with a third object, they are in equilibrium with each other (they have the same temperature)
Zeroth law of thermodynamics
energy can be converted from one form to the other, but cannot be created or destroyed
First law of thermodynamics
processes that will occur without energy
Spontaneous processes
processes that require energy input to occur
Non-spontaneous processes
chemical potential energy
Spontaneity is determined by comparing the _______________ of the system before the reaction with the free energy of the system after the reaction
reversible process
A ___________ will proceed back and forth between the two end conditions
equilibrium, free energy
Any reversible process is at _______ → no change in ___________
the difference between the sum of the internal energy and PV work energy of the reactants, and that of the products
Enthalpy change, ΔH
the difference between the randomness of the reactants and that of the products
Entropy change, ΔS
stable
Stronger bonds = more _______ molecules
released ; negative
Exothermic = energy ______, ΔH is ______
absorbed ; positive
Endothermic = energy _____, ΔH is ______
favorable ; unfavorable
The enthalpy change is _____ for exothermic reactions and ____ for endothermic reactions
a thermodynamic function that increases as the number of energetically equivalent ways of arranging the components increases
Entropy (S)
the total entropy change of the universe must be positive for a process to be spontaneous
Second law of thermodynamics
favorable ; positive
Entropy change is _____ when the result is a more random system, ΔS is ______
released ; exothermic
The entropy increase must come from heat ______ by the system; the process must be _______
positive ; favorable ; spontaneous
For a process in which the final condition is more random than the initial condition, ΔSsys is _____ and entropy change is _____ for the process to be ______
orderly ; negative
For a process in which the final condition is more ______ than the initial condition ΔSsys is _______
0
The standard enthalpy of formation, ΔHf°of an element is ___ kJ/mol
positive
The absolute entropy at 25C of an element, S° is always ________
entropy at standard conditions
S°
the amount of energy it has due to dispersion of energy through its particles
absolute entropy
for a perfect crystal at absolute zero (0 Kelvin), the absolute entropy is 0 J/(mol ∙ K)
Third law of thermodynamics
negative
A process will be spontaneous when ΔG is ______
the maximum amount of work energy that can be released to the surroundings by a system at a constant T and p
Gibbs free energy
chemical potential
Gibbs free energy is often called ________ ______ because it is analogous to the storing of energy in a mechanical system
at equilibrium
ΔG° = 0 → reaction is _____________
spontaneous
ΔG° < 0 reaction is _______
not spontaneous ; spontaneous
ΔG° > 0 reaction ____ __________ as written and is ________ in the reverse direction
the change in free energy when 1 mol of a compound forms from its constituent elements in their standard states
Free energy of formation