Chemistry - 2.2 Redox, Rusting and Iron
1/22
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
23 Terms
What is a Redox reaction?
Reactions where both reduction and oxidation take place.
Give the characteristics of an Oxidation reaction:
Gains oxygen
Loss of hydrogen
Loss of electrons
Give the characteristics of an Reduction reaction:
Loss of oxygen
Gain of hydrogen
Gain of electrons
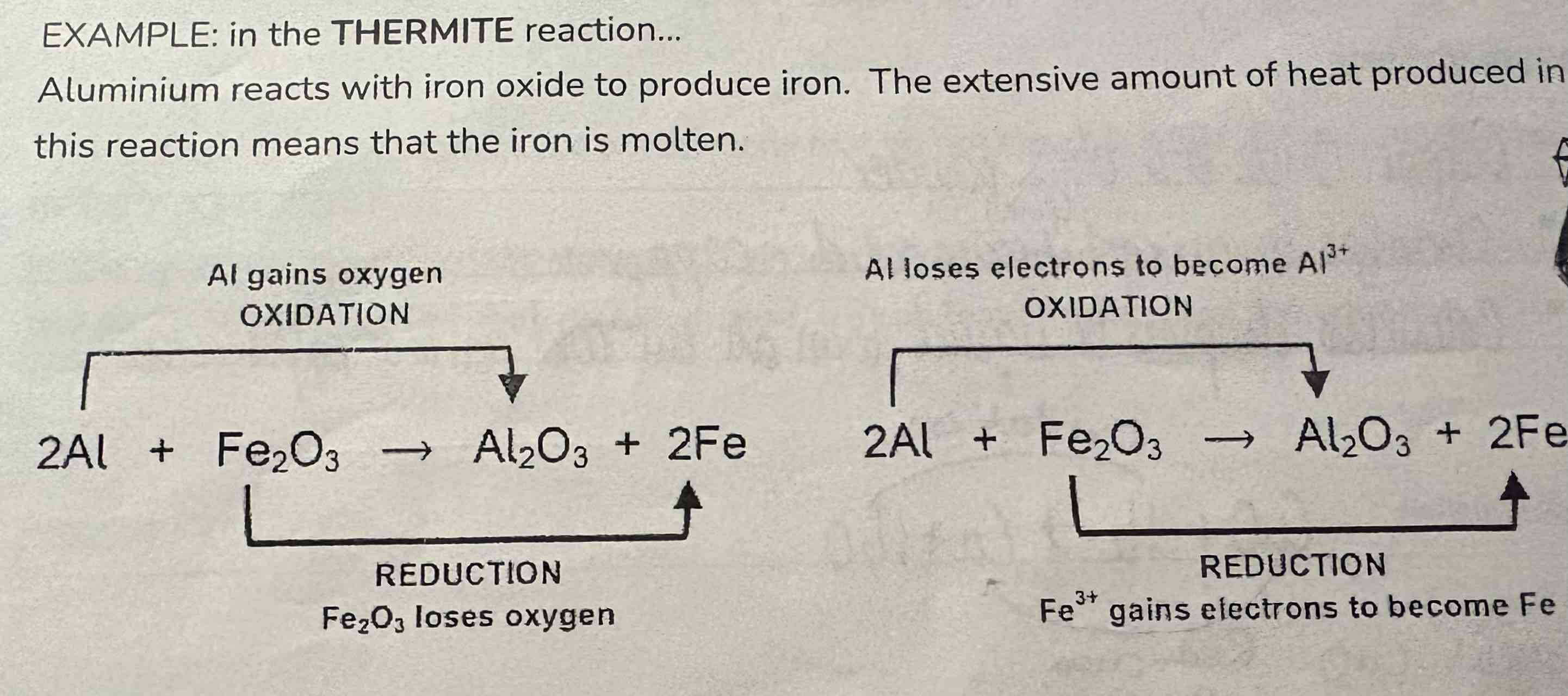
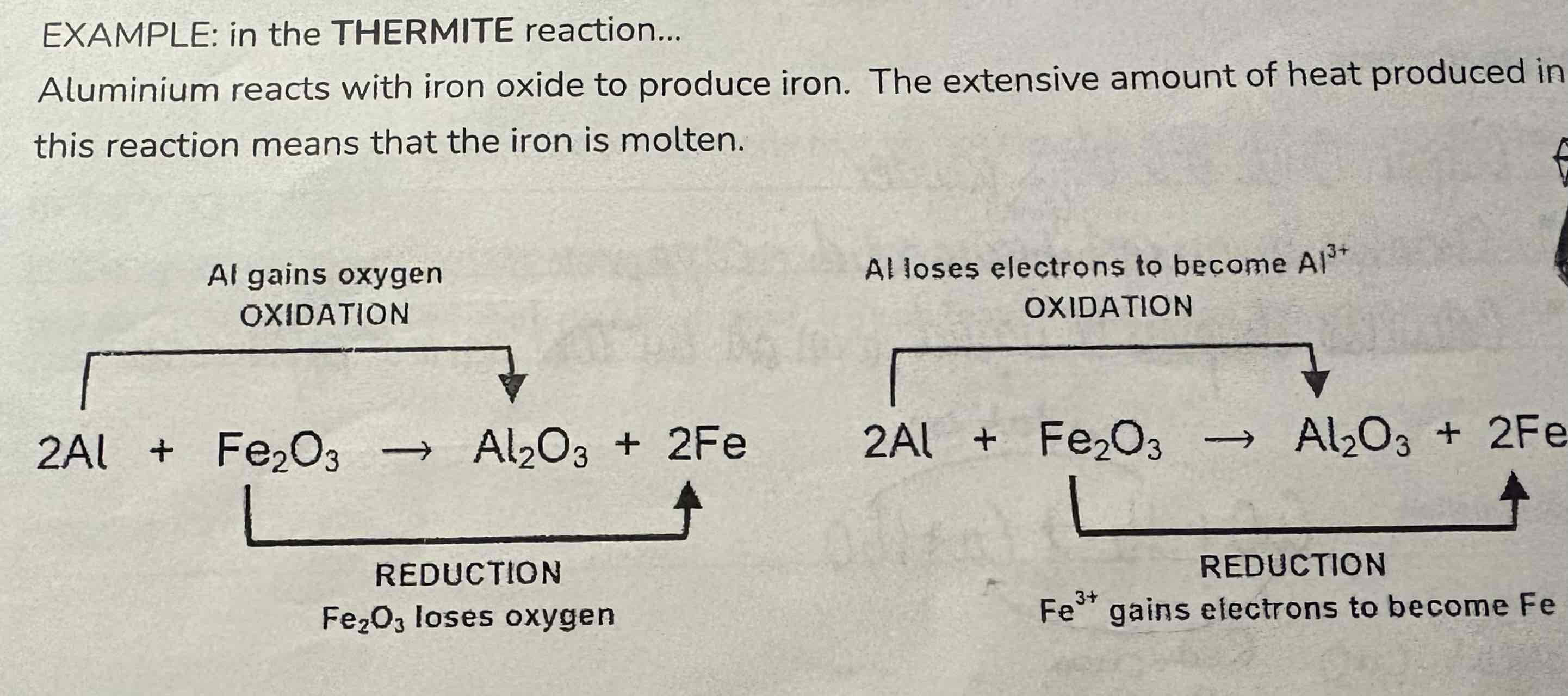
Look at this reaction:
Thermite reaction
Write out the word equation of Copper Oxide and Hydrogen:
What is the reducing agent and what is the oxidising agent:
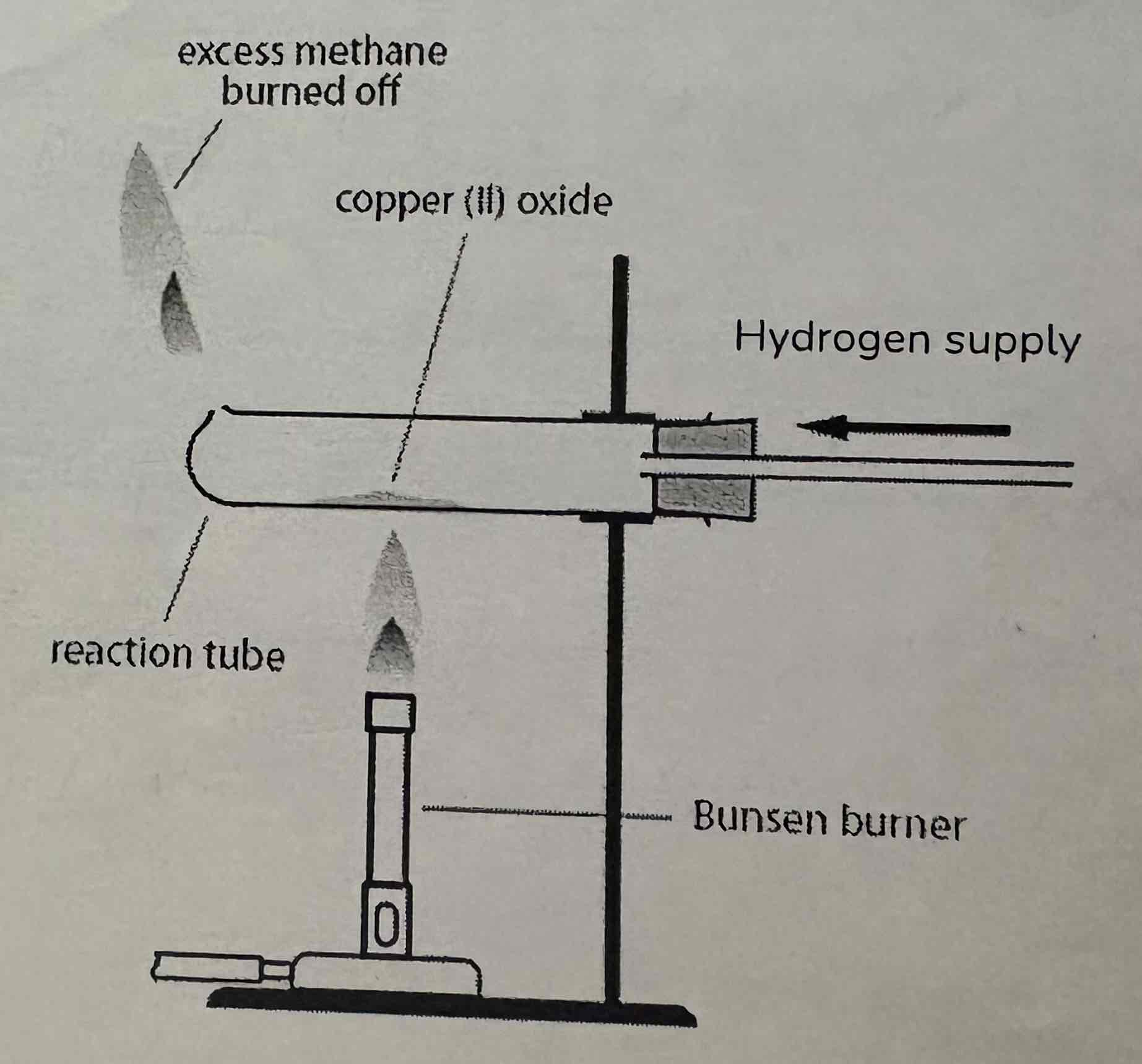
Copper Oxide + Hydrogen → Copper + Water
Hydrogen is reducing agent - It reduces the copper oxide to copper as it removes the oxygen from it
Copper Oxide is oxidising agent - The hydrogen is gaining oxygen as water is formed
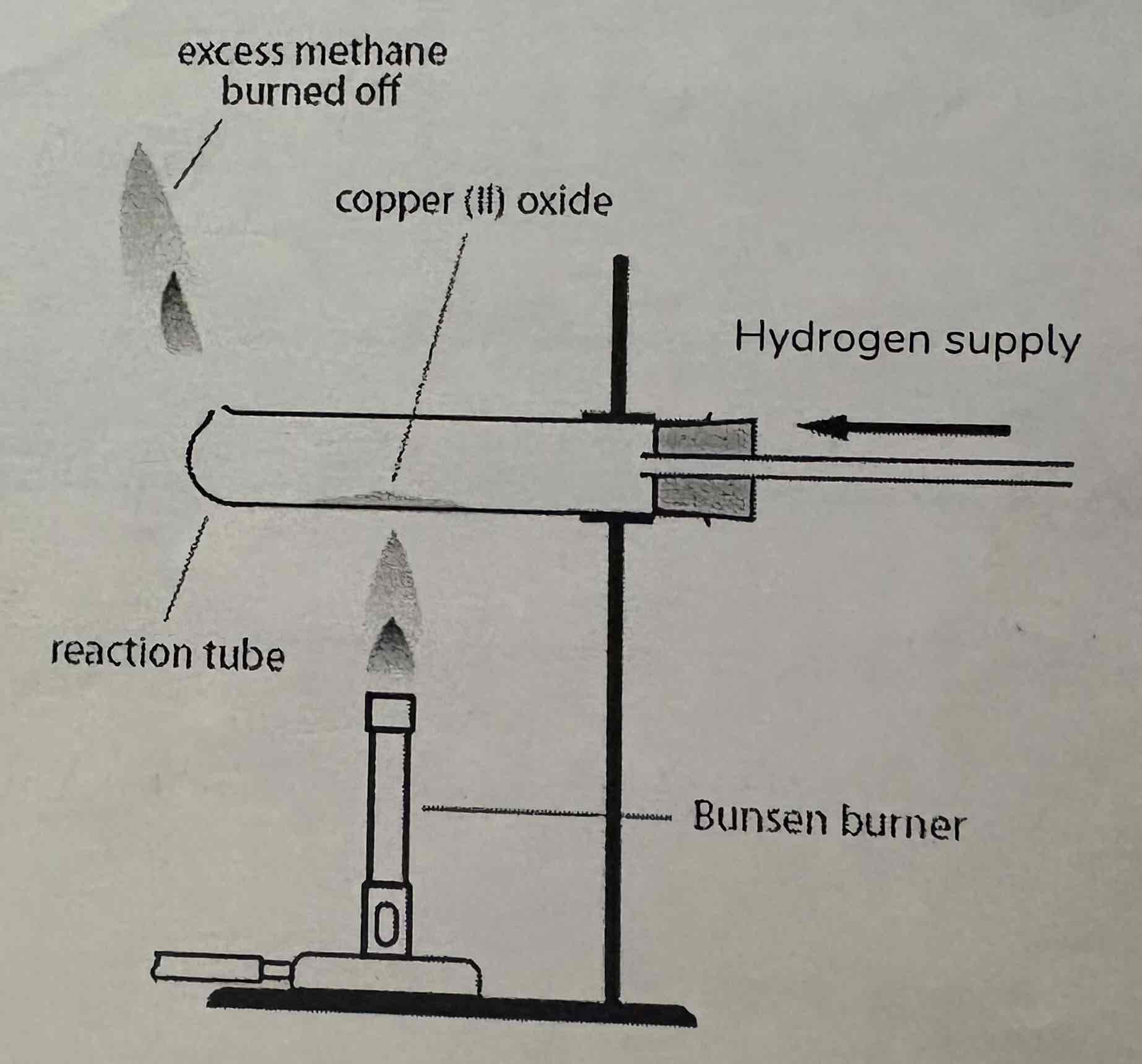
What are the observations of this reaction?
Copper Oxide is a black powder
Changes to a red-brown solid = copper
Colourless droplets of liquid form on the cold parts of apparatus
Write 2 half equations for each redox reaction:
2Al + Fe2O3 → Al2O3 + 2Fe
Use the half equations to form an ionic equation:
Oxidation:
Al → Al3+ + 3e-
Reduction:
Fe3+ + 3e- → Fe
Ionic:
Fe3+ +Al → Fe + Al3+
Explain how this reaction is described as a redox reaction:
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Cu atoms lose electrons to form Cu2+ → Oxidation
Ag+ ions gain electrons to form silver atoms → reduction
What is formula for rust and its name?
Rust:
hydrated iron(III) oxide
Fe2O3.nH2O
What is the purpose of the n?
A number which varies depending on the sample
Instead of rusting, Zinc and some other metals do what?
Corrode
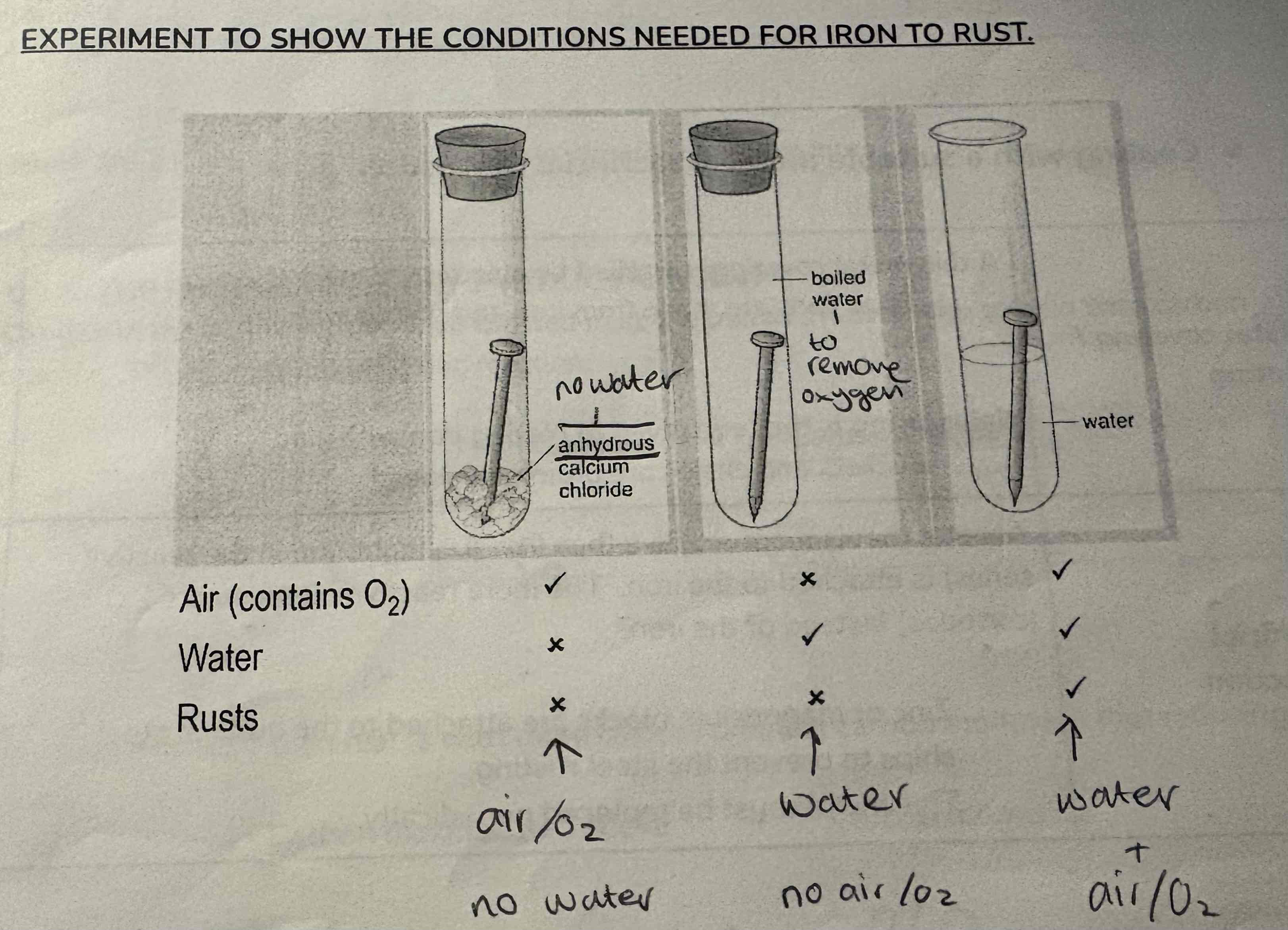
What three things are needed for rust to occur?
Iron
Oxygen
Water
What colour is rust?
Red-brown flaky solid
What type of reaction is rusting?
Oxidation reaction as the iron gains oxygen forming iron oxide
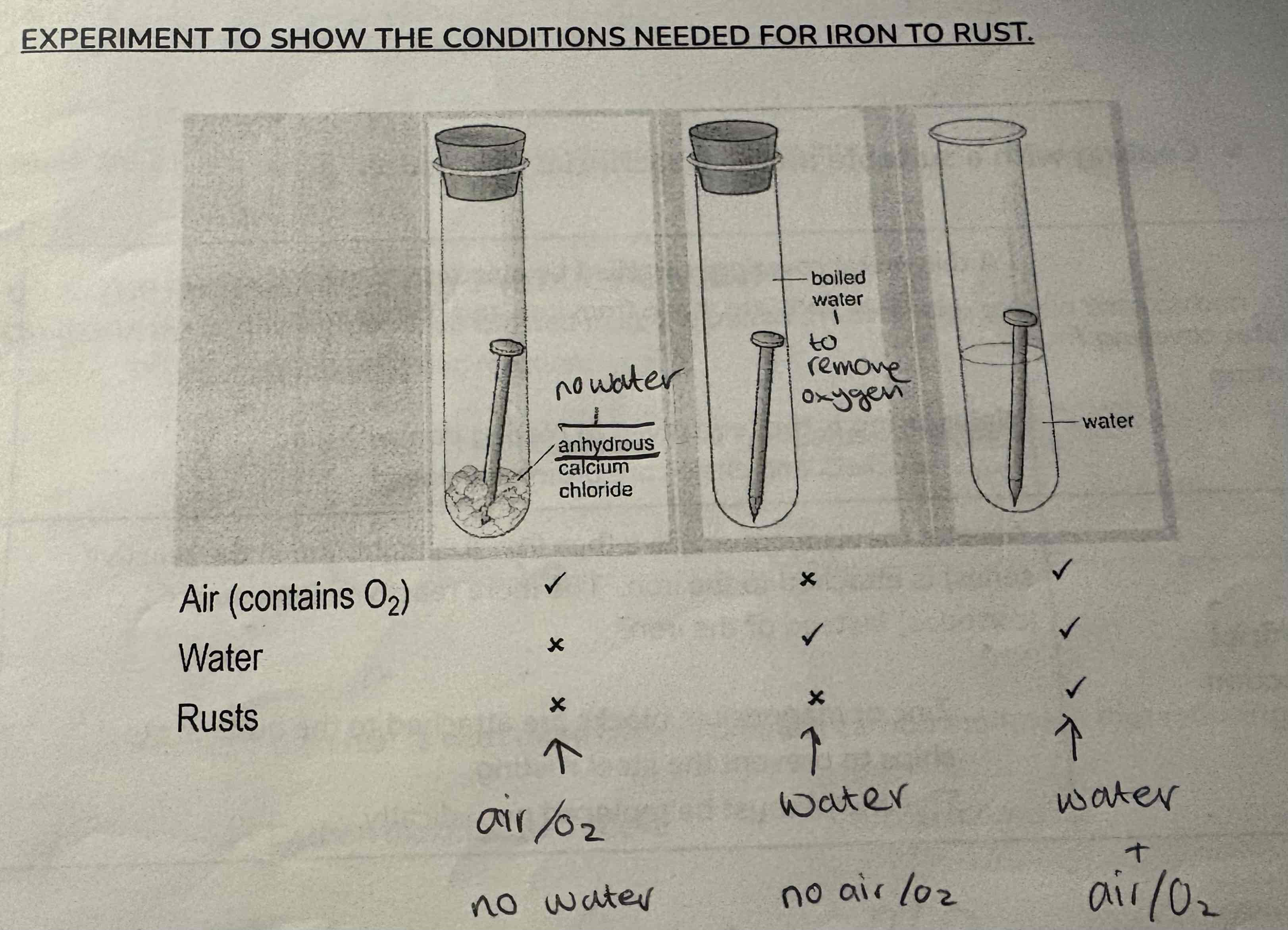
What does this experiment show?
The conditions needed for rusting to occur
List barrier methods used to prevent rusting:
Painting -
Use to protect bridges, railings, cars etc.
Oiling -
Moving parts of objects such as bicycle chains and hinges are oiled to prevent rusting
Plastic coating -
Used to cover garden furniture, fridge and dishwasher shelves
List other methods used to prevent rusting:
Metal covering/ Plating -
A thin metal covering is applied by electroplating. Food cans are made from iron and plated with tin
Galvanising is the term used when plating iron with zinc. Buckets and chains are often galvanised
Sacrificial Protection -
A metal that is more reactive than iron is attached to the iron. The more reactive metal reacts instead of the iron e.g. Zinc or magnesium blocks are attached to the hulls of steel ships to prevent the steel rusting. This metal must be replaced periodically
What is the main ore of iron
Haematite
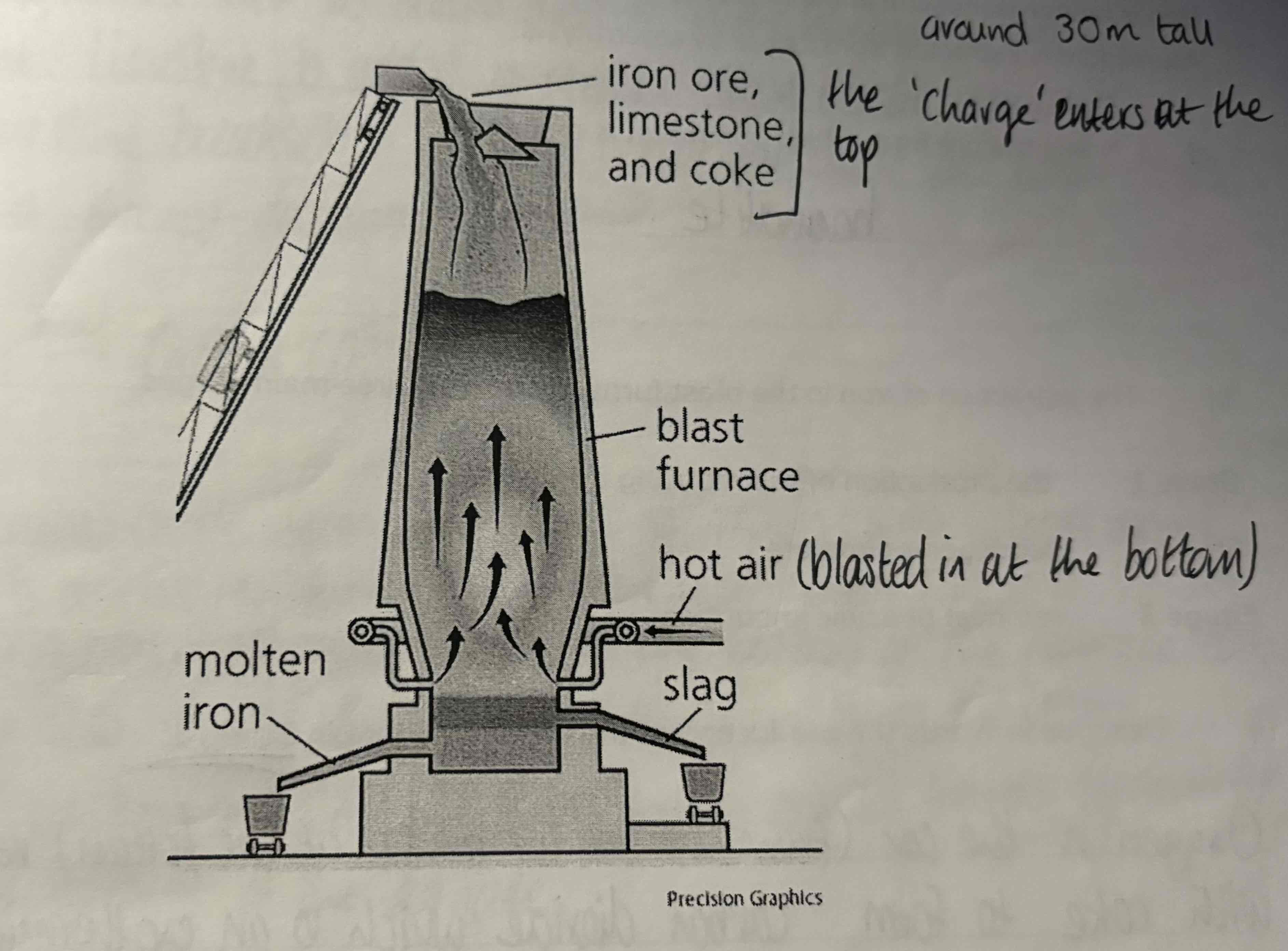
List the ‘charge’ or raw materials placed at the top of the blast furnace and the item that is pumped in at the bottom:
Charge:
Haematite
Coke - (Carbon, C)
Limestone - (Calcium Carbonate, CaCO3)
Bottom:
Hot air
What is stage 1 of extraction of Iron from Haematite?
The production of the reducing agent:
Oxygen in the hot air reacts with coke to form carbon dioxide. This is a very exothermic reaction.
C + O2 → CO2
The carbon dioxide then reacts with more coke to produce carbon monoxide which is the reducing agent
CO2 + C → 2CO
What is stage 2 of extraction of Iron from Haematite?
The reduction of haematite:
The carbon monoxide reduce the iron in the ore to give molten iron. The molten iron sinks to the bottom of the Blast Furnace and is tapped off
Fe2O3 + 3CO → 2Fe + 3CO2
What is stage 3 of extraction of Iron from Haematite?
Removal of the acidic impurities:
Acidic impurities such as silicon dioxide from the sand in the haematite rock are present. Limestone is added to remove these acidic impurities. The limestone breaks down to form calcium oxide and carbon dioxide. This is a thermal decomposition reaction which is the breaking down of compound using heat.
CaCO3 →(Heat)→ CaO +CO2
The calcium oxide formed is basic, so reacts with the silicon oxide (sand) which is acidic to form slag (calcium silicate) which is a neautralisation reaction.
CaO + SiO2 → CaSiO3
The slag (calcium sulfate) falls to the bottom of the furnace, but as it is less dense than the iron it floats on top of the iron. The molten iron and molten slag are then tapped off as liquids, separately from the bottom of the furnace
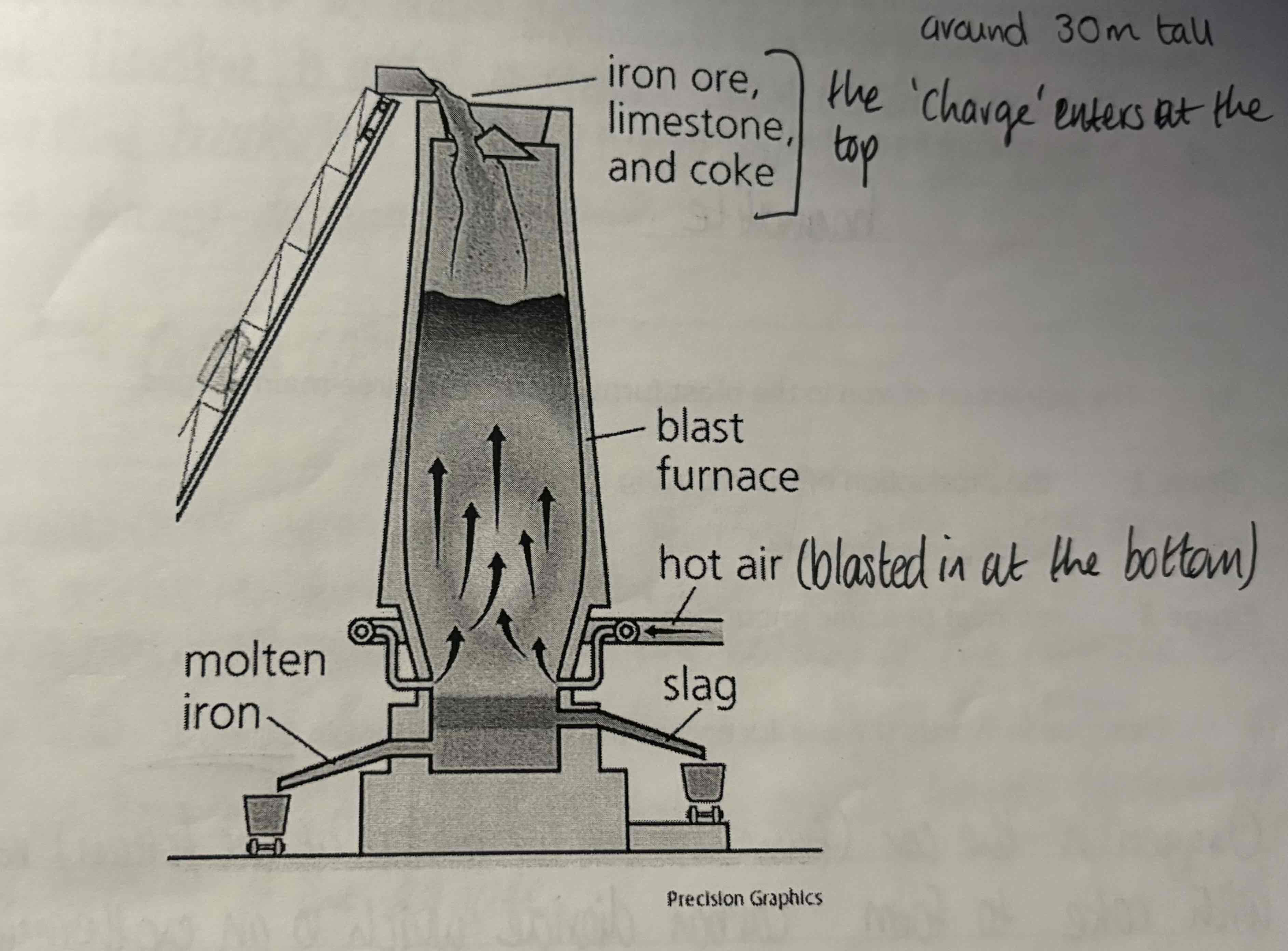
Look at this diagram of this blast furnace