Mocks flashcards ( aka topic 3, 4 , 6, 7 , 8 , 9
0.0(0)
Card Sorting
1/20
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
1
New cards
what is a neutralisation reaction
A neutralisation reaction is a reaction between an acid and a base. Acids in solution are sources of hydrogen ions , H. + alkalis in solution are sources of hydroxide ions, OH.
2
New cards
write the ionic equation for the neutralisation reaction of hydrochloric acid with potassium hydroxide
H+ + 0H- → H2O
3
New cards
what is the ammonia from the Haber process used for
nitrogen based fertilisers
4
New cards
where is nitrogen from
the air
5
New cards
where is hydrogen from
you make it from hydrocarbons like methane
6
New cards
is the Haber process endo/exo thermic
exothermic
it produces heat
it produces heat
7
New cards
what are the conditions for the haber process
450\*C
200 atmospheres
gasses pass over an iron catalyst
200 atmospheres
gasses pass over an iron catalyst
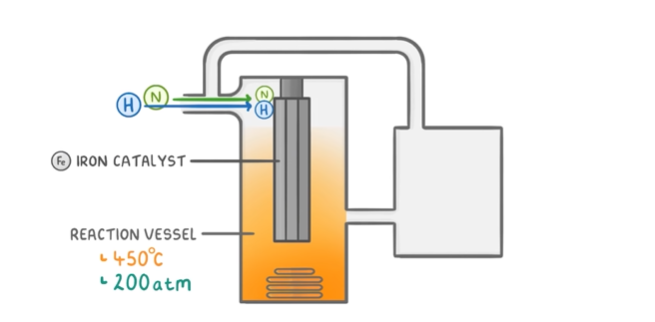
8
New cards
how is the ammonia separated from the nitrogen and hydrogen that hasn’t reacted
passing the mix thru the condenser, to cool the gaseous ammonia till it cools into liquid ammonia as it has a lower boiling point
9
New cards
what happens to the gaseous nitrogen and hydrogen when it doesn’t condense in the cooling chamber
it’s recycled
10
New cards
why do we NEED a low temp for the Haber process
the reaction is exothermic, so we need a low temp to favour -the forwards reaction → higher % yield
11
New cards
why is it not possible to have such a lower temp for the Haber process
we need a high temp for a higher rate of reaction, so 450\*C is a compromise
12
New cards
why is a high pressure needed for the haber processor
there are fewer molecules of products than reactants
so high pressure will push equilibrium to the right
high pressure also increases r8 of reaction
so high pressure will push equilibrium to the right
high pressure also increases r8 of reaction
13
New cards
what limits how high we can make pressure in the Haber process
cost
saftey
saftey
14
New cards
how does pressure effect the equilibrium of a system
to lower the pressure, the equilibrium will move to the side with the least number of molecules
\
and vice verse
\
and vice verse
15
New cards
what does an ester bond look like
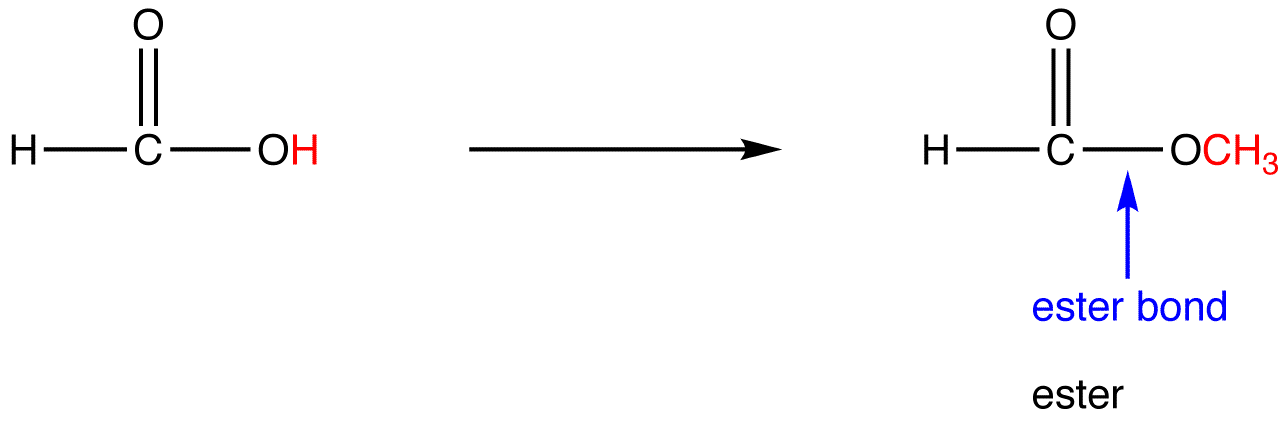
16
New cards
order of hydrocarbons in a cooling chamber
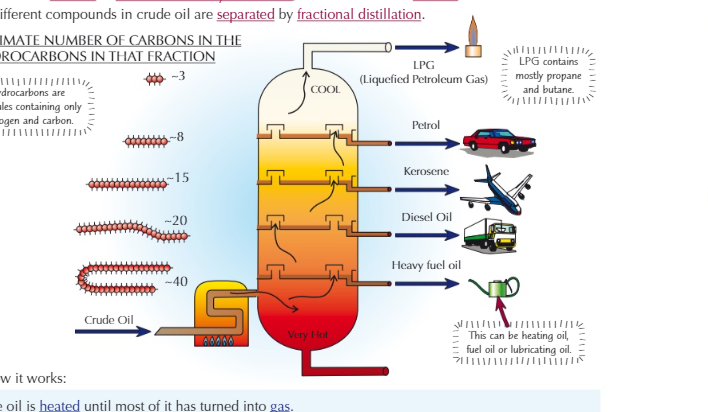
17
New cards
four ways to make something pure
filtration
distilation
crystalisation
chromatography
distilation
crystalisation
chromatography
18
New cards
pure substances melt at a specific temperature
19
New cards
meaning of affinity
Affinity is the **tendency of a chemical species such as an atom or molecule to react with another to form a chemical compound**.
20
New cards
what is Rf
The ratio of the distance moved by a compound (centre of spot from origin) to the distance moved by the solvent can be expressed as its Rf value
21
New cards
what is a thermosoftening polymer