Chapter 1: Carbohydrates
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Features of monosaccharides (CH2O)n
Linear forms have free carbonyl (C=O) group → reducing sugars
Small in size, have multiple hydroxyl (OH) groups which can form H bonds with water → readily soluble in water
Ring structures exhibit a- and b- isomerism
Examples of monosaccharides
Glucose, galactose, fructose
Formula of dissacharides
Cn(H2O)n-1
Features of disaccharides
Made up of 2 monosaccharides joined by a glycosidic bond formed between 2 monosaccharides by a condensation rxn that involves the loss of a water molc
can be split into component monosaccharides via hydrolysis rxn, where a glycosidic bond can be broken with the addition of a water molecule
Have any OH groups which extend out of the ring → form H bond with water → readily soluble in water
All are reducing sugars except sucrose
Examples of disaccharides and their constituent monomers
Sucrose (glucose + fructose), lactose (glucose + galactose), maltose (glucose + glucose)
Polysaccharides (C6H10O5)n are made up of many monosaccharides joined by ___ bonds formed between them by ___ reactions which involve the loss of ___ ___.
Glycosidic, condensation, water molecules
Starch is made up of 20% ___ and 80% ___.
Amylose, amylopectin
What is the function of starch?
Plant storage molecule
Starch is stored as granules in ___.
Chloroplasts
Starch is made up of ___ monomers
a-glucose
What are the bonds between the monomers in starch?
Amylose: a(1-4) glycosidic bonds
Amylopectin: a(1-4) glycosidic bonds within branch, a(1-6) glycosidic bonds at branch points
Structure of amylose and amylopectin
Amylose is a helical molecule while amylopectin is a helical and branched molecule
Orientation of starch molecules
All glucose monomers in the chain have the same orientation
Function of glycogen
Animal storage polysaccharide
Glycogen is stored in the ___ and ___ cells
Liver, muscle
Glycogen is made up of ___ monomers
a-glucose
Bonds between glycogen monomers
a(1-4) glycosidic bonds within branch, a(1-6) glycosidic bonds at branch points
All glucose monomers in glycogen in the chain have the ___ orientation.
Same
Structure of glycogen
Helical, more extensively branched than amylopectin
Function of cellulose
Plant structural polysaccharide
Cellulose is found in the ___ ___ of plants
Cell walls
Monomers of cellulose
B-glucose
Bonds between cellulose monomers
b(1-4) glycosidic bonds
Orientation of cellulose monomers
Alternate B glucose monomers are rotated 180 degrees with respect to each other
Structure of cellulose
Long, straight chain
Bonds between OH molecules in cellulose form…
OH groups projecting outwards in both directions allow interchain hydrogen bonding between cellulose molecules that are parallel to each other → form microfibrils
Why do the structures of starch and glycogen make them good energy storage molecules?
Helical molecules, arrangement allows many a-glucose monomers to be packed per unit volume → compact energy store
Most OH groups involved in intramolecular H bonding within the helix → few OH groups available for H bonding with water → insoluble in water, water potential unaffected by their presence
Branched → multiple branch ends which hydrolytic enzymes can work on → more glucose molecules can be released rapidly at the same time → more ATP can be generated by respiration per unit time
Large molecules → insoluble in water
Why the structure of cellulose makes it a good structural molecule
Alternate glucose monomers are rotated 180 deg wrt eo → long, straight molecule with OH groups projecting out in both directions → interchain H bonding between cellulose molecules parallel to eo → microfibrils → high tensile strength
Most OH groups involved in interchain H bonding → few OH groups available for H bonding with water → insoluble in water
Meshwork of microfibrils that form the cell wall
have a porous structure → cell freely permeable to water and solutes → allow movement of substances across cell wall
strong and rigid, distribute stress in all directions to prevent plant cells from bursting due to osmotic stress
Cellulases that hydrolyse cellulose are found in very few organisms → cellulose cannot be hydrolysed by most organisms and be used as respiratory substrate —> good structural molecule
Benedict’s test for reducing sugars
Place 2cm³ of test solution in a test tube
Add equal volume of Benedict’s reagent
Shake mixture
Heat by immersing tube in boiling water bath for 3-4 minutes
Brick-red ppt → reducing sugar is present
Test for non-reducing sugars
If a negative result for BT is obtained for test solution, then
boil equal volume of test solution with dilute HCl for ~1min → hydrolyse disaccharide to monosaccharides
Cool contents of tube
Neutralise the acidic content with sodium bicarbonate solution
Carry out Benedict’s test for reducing sugar
Presence of non-reducing sugar indicated by:
Blue solution remains when BT is first carried out
After acid hydrolysis, BT carried out again → colour of final suspension depends on amount of sugar present
Iodine test for starch
Add a few drops of iodine solution to 1cm³ of test solution
Observe any colour change (blue-black → starch present, orange → starch absent)
Cellulose has a structural function while starch has a storage function. Relate these functional differences to the differences in molecular structure of cellulose and starch. [4]
Cellulose has B-glucose monomers that are linked by B(1-4) glycosidic bonds: enzymes that hydrolysis these bonds are rarely found in nature and therefore likely to remain intact → suitable as structural molc
Cellulose alternate glucose residues inverted 180˚ wrt one another allowing straight chains to be formed with hydroxyl groups projecting out in either direction. Numerous H bonding b/w adjacent cellulose molcs form microfibrils: straight chains allow packing of cellulose chains into bundles of microfibrils with high tensile strength that make up cell wall
Starch has A glucose molecules that are linked by a(1-4) glycosidic bonds. Enzyme that hydrolysis these bonds are commonly available → glucose units readily released for respiration to yield energy
Starch A glucose monomers are linked by a(1-4) glycosidic bonds which give rise to helical molecules of amylose as each residue in bent in one direction wrt adjacent molc: helical arrangement allows more glucose residues per unit volume → compact storage molc
Suggest why amylase (hydrolyse starch) will not catalyse the hydrolysis of cellulose. [2]
Amylase has a specific active site with complementary charge and conformation to starch which it binds and catalyses the hydrolysis of a(1-4) glycosidic bonds
Cellulose has B(1-4) glycosidic bonds whose 3D conformation is not complementary to AS of amylase
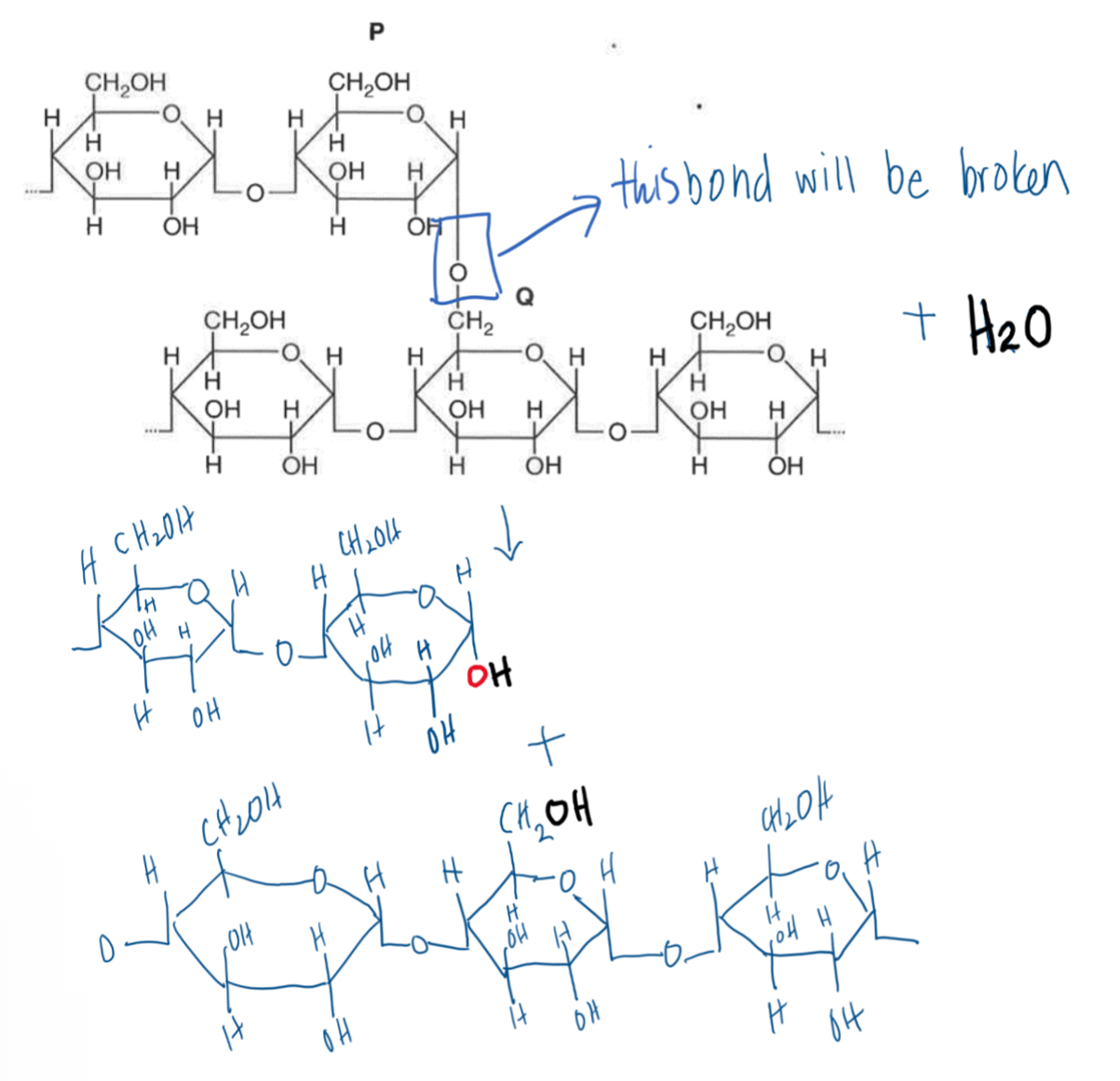
Use an annotated diagram to show how the bond between P (main branch of glycogen) and Q (branch) is broken
Suggest why cellulose must be synthesised at the cell surface membrane and not inside the cell. [3]
Cellulose is a macromolecule found outside the cell as part of the cell wall therefore it is easier to deposit it there
Cellulose molecule may be too large to be transported through the cell membrane if it has to be transported to the exterior of the cell
Cellulose is insoluble in the hydrophobic core of the lipid bilayer, hence synthesised at the cell surface membrane and transported out of the cell
Identify one property of starch that could account for its stability over long periods of time. [1]
Large molecule and insoluble in water, hence will not participate in metabolic reactions in aq medium of the cell
Explain how cellulose differs from starch in function
