MMSC415 Exam 2
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
94 Terms
What are the five types of vaccines?
- Killed microorganisms or inactivated viruses --> Killed with heat, chemical treatment, or radiation; Downside: incomplete inactivation can cause disease when introduced into the patient
- Live attenuated vaccines --> Mutant form of live pathogen that grows poorly in human cells or is no longer pathogenic; Downside: can convert to wild type and infect people or revert to pathogenic form and spread to others; Attenuated by growing the virus in another species so it will become specialized for those cells; attenuated = weakened
- Subunit vaccines --> Purified single or mixed components; ex. Proteins and polysaccharides → pick the most immunogenic components of pathogens; Ex. toxoids (inactivated toxins) like diphtheria and tetanus; acellular pertussis (toxin + protein)
- Conjugate vaccines --> Protein-polysaccharide complex; Sometimes polysaccharides don't activate T cells so linking it to a protein will ensure T cell activation so they can form memory cells
- Recombinant vaccines --> Gene cloned into another microorganism in vitro; Process: 1. Identify the target subunit 2. Isolate gene and transfer into bacteria or yeast 3. Culture them and harvest 4. Extract and purify subunit
Difference between immediate and delayed hypersensitivity reactions
- Immediate --> antibody mediated; occurs within seconds to hours of second contact with allergen; Types I-III
- Delayed --> cell-mediated; >12 hours, max 48-72 hours; type IV
Type I hypersensitivity
- allergen elicits an IgE antibody response upon sensitization
- Exposed to allergen, activation of antigen-specific Th2 cells, Th2 cells activate B cells to secrete IgE, mast cells and circulating basophils and eosinophils bind allergen specific IgE via the Fc portion to their surface using receptors, cells (majority mast cells) are now armed to recognize a secondary exposure
What do mast cells and basophils contain in their granules
histamine, heparin, proteases
Early vs late phage type I hypersensitivity
- Early: mast cells
- Late: eosinophils
Examples of type I hypersensitivity
Asthma, dermatitis, hives, angioedema
Diagnosis for Type I hypersensitivity
- intradermal testing
- Radioallergosorbent test (RAST) --> tests for antigen specific IgE
- Radioimmunosorbent test (RIST) --> measures total serum IgE
- food elimination diet
- oral food challenges
Treatment for type I hypersensitivity
- avoid allergens
- immunotherapy --> allergy shots to desensitize immune system and hopefully develop tolerance
- treat symptoms
Type II hypersensitivity
- cytotoxic
- Self reactive B and T cells escape positive and negative selection → reactive to intrinsic antigens
- Ex. drug induced → 1. Penicillin binds to cell surface receptor causing it to appear as foreign 2. B cell encounters the antigen, internalizes it, and starts producing antibodies against it with the help of a T cell 3. Antibodies are produced (IgM or IgG) 4. Antibodies bind to the epitope and opsonization with complement components occurs and lysis is attempted via MAC complex 5. Antibody-dependent cell mediated cytotoxicity via NK cells, macrophages, neutrophils, and eosinophils calling
Examples of Type II hypersensitivity reactions
- acute hemolytic transfusion reactions
- hemolytic disease of the newborn
- drug induced hemolytic anemia
- myasthenia gravis
Type III hypersensitivity
- immune complex
- Occurs through the deposition of circulating immune complexes (antigen-antibody complexes) in tissues; accumulate in the skin, joints, blood vessel walls, lung alveoli, and kidney glomeruli
- Immune complexes are usually cleared by monocyte-macrophage system but small complexes are not as attractive and may continue to circulate and then deposit in various tissues
- PMNS attempt to phagocytize immune complexes that are bound to tissues but they can't so they degranulate and release toxic granules leading to tissue damage
Examples of type III hypersensitivitiy
- arthus reaction
- serum sickness
- rheumatoid arthritis
- SLE
- farmers lung
Type IV hypersensitivity reaction
- DTH --> delayed-type hypersensitivity
- T cells
- 1. Initiated by CD4+ T cells when they recognize and bind foreign antigen presented with MHCII by APCs → APC secretes IL-12 causing TH0 cells to differentiate into TH1 cells 2. TH1 cells secrete IL-2 causing proliferation and IFN-y recruits secondary cells like macrophages, neutrophils, and other T cels 3. Phagocytes release proinflammatory cytokines and degranulation causes cellular tissue damage
- Antigens inside cells are presented with MHCI to CTLs which kills target cells with perforins and granzymes
Examples of Type IV hypersensitivity
- granulomatous
- TB skin test
- contact dermatitis
B cell deficiencies
- absent, low in numbers, decreased Ab production, heavy or light chain deficiencies, altered function
- X-linked agammaglobulinemia → defect in tyrosine kinase does not allow B cells to mature; low levels of B cells and plasma cells and low/abcense of antibodies
- Selective IgA deficiency → most common primary antibody deficiency as no class switching is occurring; common to have recurrent upper respiratory tract infections
T cell deficiencies
- T cells → absent, low in numbers, altered function
- X-linked hyper IgM syndrome → defect in CD40L on T cells; high levels of IgM and low levels of IgG, IgA, and IgE
- Wiskott-Aldrich Syndrome → WAS gene mutation leads to nonfunctional WAS protein; loss of/impairment of cell signaling and actin cytoskeleton function, causing immunodeficiency, thrombocytopenia (low platelet count), and eczema
- DiGeorge syndrome → thymus fails to develop so T cells are affected
- Adenosine deaminase deficiency (ADA) → purine degradation leading to the accumulation of toxic deoxyadenosine in human cells which is toxic for developing T cells
Wiskott-Aldrich Syndrome
Type of T cell immunodeficiency --> WAS gene mutation leads to nonfunctional WAS protein; loss of/impairment of cell signaling and actin cytoskeleton function, causing immunodeficiency, thrombocytopenia (low platelet count), and eczema
Severe combined immunodeficiency (SCID)
family of disorders resulting from a defect in lymphocyte development that alters either T cells or T and B cells
Example of a disease that arises from the immunodeficiency of phagocytic cells
Chronic granulomatous disease: defective NADPH oxidase → no ROS, meaning neutrophils can engulf but not destroy
Types of immunodeficiencies
B cell, T cell, SCID, complement, phagocytic cell, ataxia-telangiectasia
Complement immunodeficiency
- decreases immune complex clearance
- Deficiency of C1 inhibitor produces hereditary angioedema → inhibits the activation of the C1 complex, producing an uncontrolled classical pathway leading to episodes of swelling, predominantly in the face
Ataxia-telangiectasia
- ataxia = poor coordination, telangiectasia = small dilated blood vessels
- Mutations in ATM gene lead to inadequate/no response to DNA double-strand breaks
- Immunodeficiency in developing lymphocytes and impaired VDJ recombination
ELISA
Detect extractable nuclear antigens (ENAs)
ANA trends based on immunodeficiency
- homogeneous --> SLE
- systemic sclerosis --> centromere
- Sjogren's syndrome --> speckled
What are the possible ANA trends?
homogeneous, speckled, nucleolar, nuclear membrane/peripheral, centromere
what is autoimmunity
loss of self tolerance
what hypersensitivity reactions to autoimmune diseases resemble
- type II, III, and IV
- type II --> autoantibodies to host cell surface or extracellular matrix
- type III --> soluble immune complexes deposit in tissues
- type IV --> autoimmunity caused by effector T cells
Systemic lupus erythematosus pathology
Increased TH cells and decreased Treg cell function, overreactive B cells, immune complexes are formed and not cleared, and circulating autoantibodies (IgG) to specific cellular components common to many different cell types
Systemic lupus erythematosus diagnosis/serology
antinuclear antibodies (ANA), anti-dsDNA antibodies, anti-smith antibodies, low complement levels
Systemic Sclerosis
- increased collagen (structural protein in skin and other connective tissues) production, deposited in skin and other organs
- centromere ANA pattern
Sjogren's syndrome
- autoimmune disorder against lacrimal and salivary glands leading to dry mouth and eyes
- speckled ANA pattern
Rheumatoid Arthritis pathogenesis
stimulation of B cells that make IgM, IgG, or IgA against the Fc region of IgG and these autoantibodies are called Rheumatoid factor
Rheumatoid Arthritis diagnosis
- Rheumatoid factor
- anti-CCP
multiple sclerosis pathogenesis
- activated T cells surmount the blood-brain barrier to secrete cytokines
- EBV mimics myelin basic protein in the myelin sheath via molecular mimicry
Myasthenia gravis pathogenesis
neuromuscular junction releases acetylcholine which binds to acetylcholine receptor → autoantibodies to AChR
Diabetes Mellitus (Type I) pathogenesis
selective destruction of the insulin-producing beta cells in the pancreas; hypothesis is that T cells lose tolerance to beta cells, THi cells develop and trigger CMI response; autoantibodies are present but do not seem to play a role in the damage
Autoimmune thyroid diseases
- Hashimoto thyroiditis --> autoantibodies and TH17 cells specific for thyroid antigens; thyroperoxidase antibodies (anti-TPO); formation of a goiter, hypothyroidism caused by binding of antibodies that interfere with the production of thyroid hormones
- Graves disease --> autoantibodies to thyroid-stimulating hormone receptor cause thyroid stimulation → hyperthyroidism
Ankylosing Spondylitis
chronic inflammation of the spine beginning in the sacroiliac joint
Risk factor of Ankylosing Spondylitis
HLA-B27
Goodpasture syndrome
anti-glomerular basement membrane disease affecting the kidneys and lungs; Anti-GBM antibodies
Autimmune hemolytic anemias
- Cold agglutinin disease (CAD) → IgM to human RBC I antigen
- Paroxysmal cold hemoglobinuria (PCH) → IgG coats RBCs at cold temp and fixes complement, but lysis of RBC occurs upon return to 37℃
- Warm autoimmune hemolytic anemia → IgG coats RBCs at 37℃ and cells are lysed
Multiple myeloma
- proliferation of a single B cell clone, monoclonal gammopathy
- Bence jones proteins: secreted free light chains; detained in blood and urine and are a diagnostic
Diagnosis of multiple myeloma
CRAB:
- C --> hypercalcemia
- R --> renal insufficiency
- A --> anemia
- B --> bone lesions
Waldenstrom's macroglobulinemia
form of non-Hodgkin lymphoma; high levels of circulating monoclonal IgM; causes many symptoms due to hyper viscosity syndrome
Types of grafts
- Xenograft: translation from one specific to a different species
- Allograft: transplantation from one individual to a genetically non-identical recipient (most common)
- Isograft: transplantation from one individual to a genetically identical recipient
- Autograft: transplantation from one site of an individual to another site of the same individual
Histocompatibility
determined by the compatibility of HLA genes; for solid organs you want to match ABO blood group and as many HLAs as possible (MHC-I and MHC-II are important); molecular HLA typing
Graft vs. host disease
- seen in bone marrow and stem cell transplants
- Graft attacks recipient cells and tissues systemically
What does development of graft vs host disease require
histoincompatibility, immunocompetent donor T cells, and an immunosuppressed or immunodeficient host
Tumor (immune) surveillance
theory of Paul Ehrlich; tumor cells typically possess weak immunogens and decreased expression of MHC/HLA antigens
What are the steps of immunoediting
- Elimination: innate and adaptive immune responses detect and destroy tumor cells
- Equilibrium: tumor cells are not completely eradicated, selective pressure exerted by immune cells
- Escape: tumor cells can spread to other sites and lead to clinically apparent disease
Tumor markers (antigens)
- Alpha-fetoprotein (AFP): major plasma protein produced by fetal liver/yolk sac, stops by birth; AFP-secreting tumors seen in hepatocellular carcinoma as tumors of the liver revert to old form and make abnormal antigens; elevated serum AFP
- Carcinoembryonic antigen (CEA): glycoprotein involved in cell adhesion; produced in GI tract of fetus and stops before birth; CEA-secreting tumors seen in colorectal carcinoma; elevated serum CEA
- Prostate-specific antigen (PSA): secreted by prostate gland; elevated in prostate cancer; used for screening but not for definite diagnosis
- Human chorionic gonadotropin (hCG): hormone secreted by trophoblast cells surrounding embryo; hCG secreting-tumors seen in testicular cancer
- Cancer antigens help monitor patients undergoing treatment: CA-125 → ovarian cancer; CA19-9 → pancreatic cancer
What prevents graft vs host disease
irradiation → killing of lymphocytes in the blood
Prozone effect
antibody excess
Post zone effect
antigen excess
Turbidimetry
the measurement of light transmitted through a suspension of particles; measures turbidity or cloudiness of solution
Nephelometry
a direct measurement of light scattered by particles suspended in solution
What is the second step of agglutination reactions
lattice formation
Passive agglutination
carrier particle coated with Ag not normally present; ex. Latex agglutination to detect Ab
Reverse passive agglutination
Ab attached to a carrier particle facing outward; ex. Latex agglutination to detect Ag
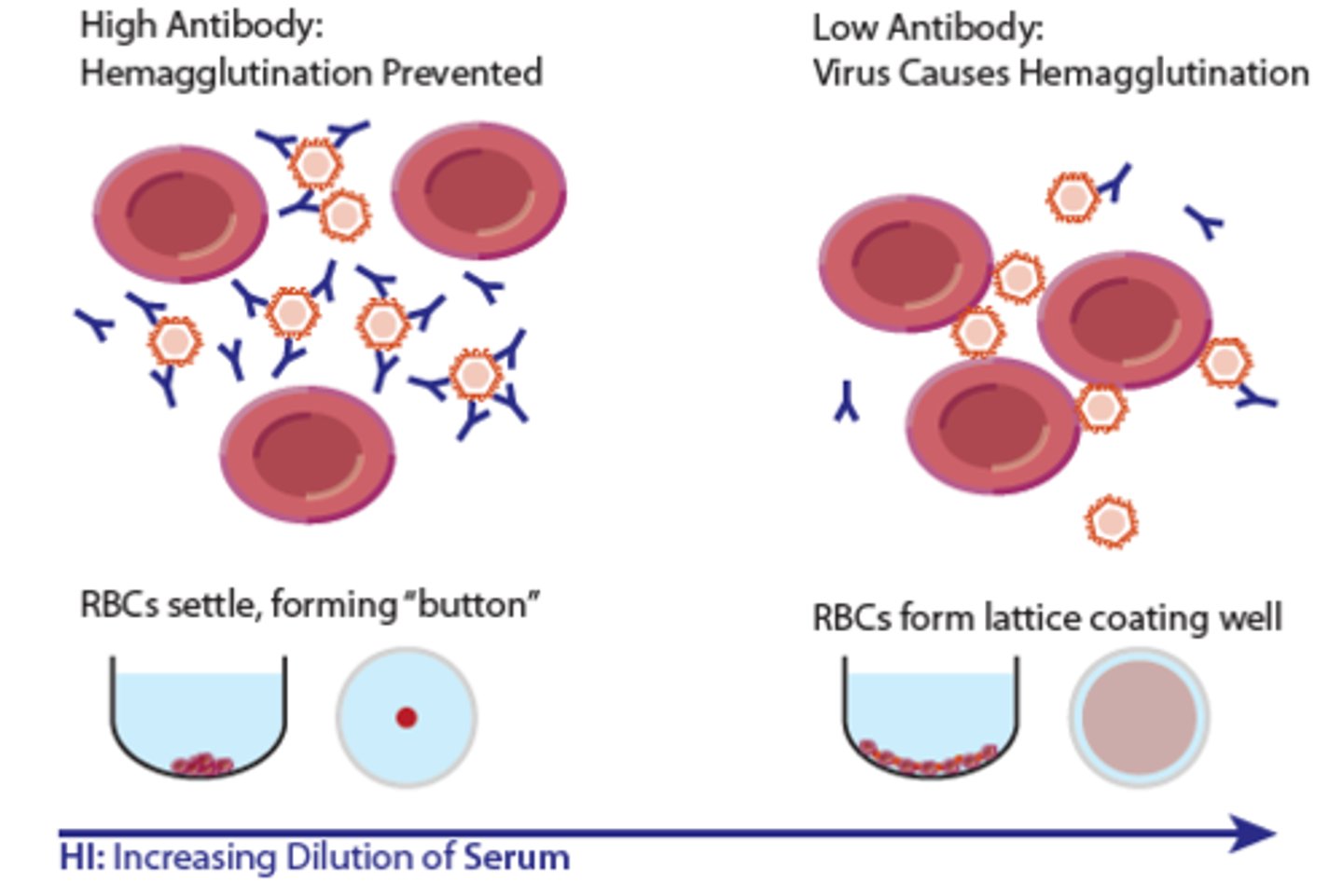
hemagglutination inhibition
some viruses naturally agglutinate RBCs; mix patient serum with viral suspension and then add the RBCs the virus is known to agglutinate; no agglutination means the patient serum had antibodies specific to the virus, which prevented hemagglutination
What are the different labels used in labeled antibody tests
fluorescent (uses fluorochromes, ex. Fluorescein, isothiocyanate), enzyme, chemiluminescent, and radioactive
What does a direct fluorescent antibody (DFA) test for
detect antigens in tissue, sample, or cell suspension on a slide using an antigen-specific fluorescently labeled Ab
What is added to the solid phase antigen in a DFA
Solid phase antigen on a slide + labeled antibody conjugate (antibody coupled with fluorochrome) → antigen-antibody combination fluorescence
Indirect fluorescent antibody (IFA) test
detect unlabeled, primary patient antibody in sample using fluorescent-labeled secondary antibody
What is added to the solid phase antigen in an IFA
Solid phase antigen on slide + unlabeled antibody (patient serum) → antigen antibody combination + labeled anti-immunoglobulin conjugate → fluorescence
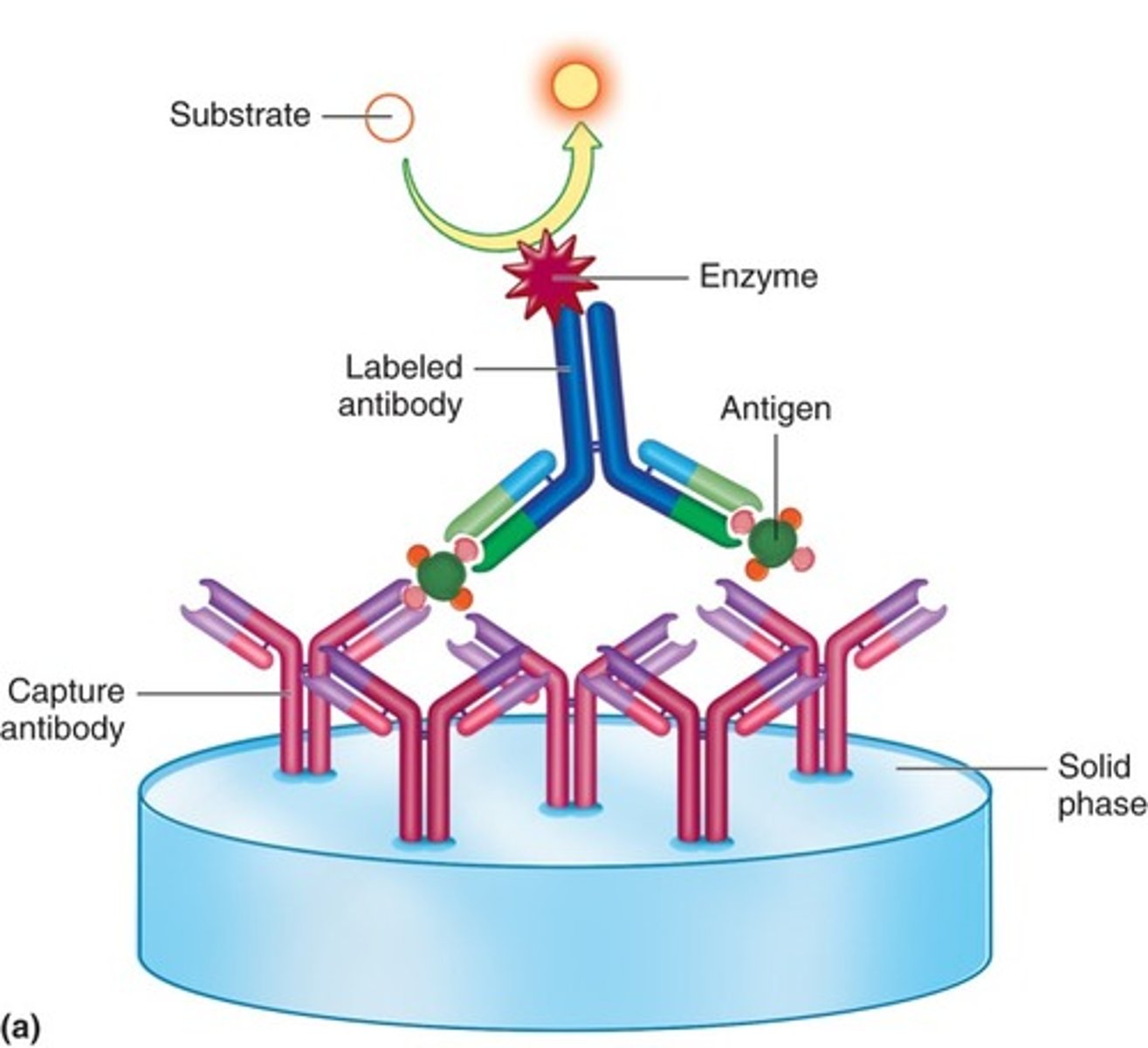
What does a capture/sandwich immunoassay detect
antigens or antibodies
Competitive immunoassay for antibody results
signal detected inversely proportional to antibody concentration
What does ELISA stand for
Enzyme-linked imunosorbent assay
What are cells in a fluorescence-activated cell sorting stained with
fluorescently labeled antibodies
what does LASER in fluorescence-activated cell sorting stand for
light-amplified stimulated emitted radiation
What does EIA stand for
enzyme immunoassay
What do you incubate patient sample with in the enzyme-multiplied immunoassay technique (EMIT)
anti-drug Ab
What does Streptavidin-biotin labeling do
amplifies signal
What does RAST stand for
radioallergosorbent test
What does RAST measure
allergen-specific IgE
What is Western Blotting
- Electrophoretically separate subspecies of antigens (proteins) and transfer them to a membrane
- Add patient serum with antibodies to specific epitopes of separated antigens
- Detect antibodies by using labeled anti-human antibodies
- Visualize color development enzymatically or by chemiluminescence
What does western blotting detect
Detect antibodies by using labeled anti-human antibodies
What does complement fixation test detect
specific antibody
What is used as a reagent in the complement fixation test
complement
What do results of a complement fixation test look like
if hemolysis present (pink) test is negative, but if no hemolysis (RBC pellet) test is positive
What is a primary lesion in syphilis called
chancre
Describe a flocculation reaction for syphilis testing
IgG or IgM to lipids released from damaged host cells during infection; looking for antibodies indicating inflammatory process not against pathogen;
What are treponemal tests used for
confirmation of reactive RPR or VDRL
What should you do if there is blood present in a cerebrospinal fluid test for neurosyphilis
reject the sample
What is the main concern with using live-attenuated vaccines?
Viruses may revert to their pathogenic form
What is the main concern with using subunit vaccines
Purified antigens may not be immunogenic
What is the main concern with using killed/inactivated vaccines
Viruses may not be completely inactivated
What is an example of a complement deficiency?
hereditary angioedema
How could you test for FUNCTIONAL humoral-mediated immunity?
vaccinate the patient and then later test for antibody titer
Which of the following may result in a severe combined immunodeficiency?
a. DiGeorge syndrome
b. X-linked agammaglobulinemia (XLA)
c. Adenosine deaminase (ADA) deficiency
A and C
a. DiGeorge syndrome → lack of T cells because the thymus is underdeveloped
b. X-linked agammaglobulinemia (XLA)
c. Adenosine deaminase (ADA) deficiency → toxic buildup of adenosine byproducts in T cells causing death
How should serum be stored if testing will be delayed?
short-term refrigerated, long-term frozen
T/F - Hemolysis in a complement fixation test is a positive result
False - complement is available to fix RBCs if the antibody is NOT present
What is being detected in a Western blot or dot blot?
specific antibodies in a patient sample
What is molecular mimicry
structural similarities between a microorganism's antigens and our self-antigens