BIOL 111 Exam 2
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
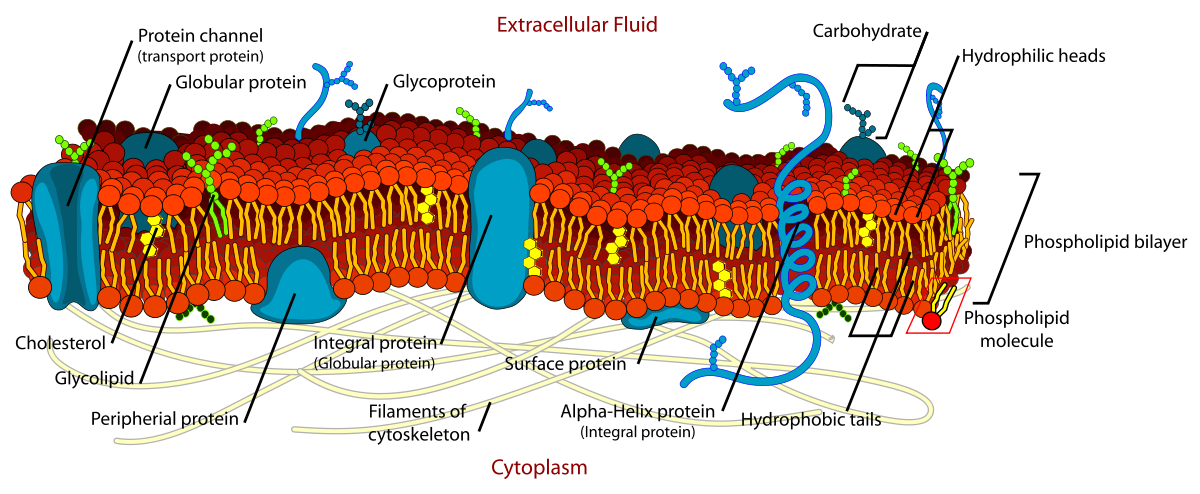
Membranes
A “collage” of different proteins embedded in the fluid matrix of the lipid bilayer
Abstract
Each individual pieces don't make sense by themselves but works when put together
Flexible but maintains structure
Plasma membrane
Dynamic and ever-changing boundary that separates the living cell from its nonliving surroundings
Exhibits “selective permeability”
Allows some substances to cross more easily
Ex: bouncer at a nightclub
Certain people in
Stop certain people
Functions to:
Define outer border of cells and organelles
Manages what enters and exits: “bounder” of the cell
Receives external signals and initiates cellular responses
Adheres to neighboring cells
Fluid mosaic model
Membrane is a fluid structure with a “mosaic” of various components embedded in it
Mixture of phospholipids, cholesterol, proteins, and carbs
Glycolipid and glycoprotein
Phospholipid: main component
Phospholipid bilayer
Protein go from external environment to internal environment
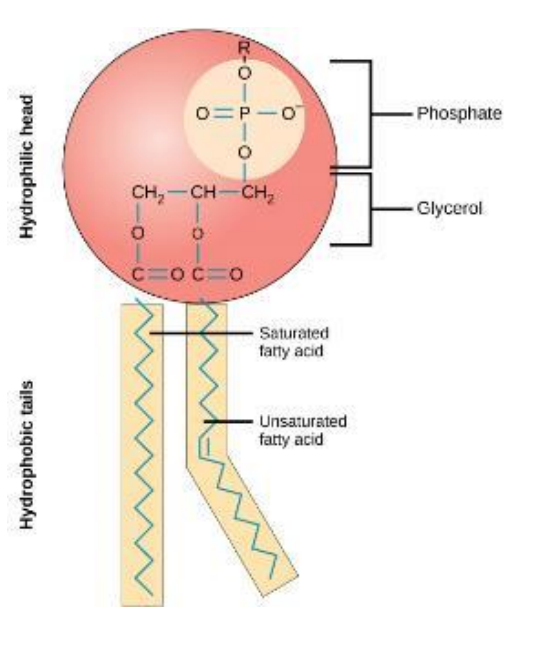
Phospholipids
Major component of plasma membrane
Amphipathic → contain both hydrophilic and hydrophobic regions
Head: loves water
Tails: hates water
Can move around within the bilayer
Movement makes it impossible to form a completely impenetrable barrier
Allow for changing environment
Fluidity of the membrane (Temperature)
Temperature → affect movement “rate” and distance
Iceberg in ocean
Colder = closer to phospholipids → restricts small molecules
Rigid; may break
Warmer = more separated phospholipids → leaves larger gaps
More flexible
May not hold the shape
Fluidity of the membrane (Cholesterol)
Cholesterol → randomly distributed across the bilayer, helping it stay fluid
Introduce some gaps
Increased fluidity at low temperatures and decreases fluidity at high temperature
More cholesterol at low temp
Less cholesterol at high temp
Acts as a buffer: keeps membranes fluid when cold and not too fluid when hot
Fix rigidity
Fluidity of the membrane (Fatty Acid)
Fatty acid composition → make up the hydrophobic phospholipid tails
Saturated fats are straight, easy to pack tight
Hot → increase saturated fat
Unsaturated fats have double bonds that create kinks in the chains, making it more fluid
Cold → increase unsaturated fat
Counterbalance swings in temperature
Proteins
Second major component of membranes
Functions include: transportation, receptors, enzymatic, binding
Ex: tollway
Integral proteins: span the entire bilayer moving things in/out
Peripheral proteins: either on exterior or interior surface
Usually enzymes or structural attachments
Don’t span both pieces of bilayer
Carbohydrates
Third major component of membranes
Only located on exterior surface of the plasma membrane and bound to something else
Bound to protein → glycoprotein
Bound to lipid → glycolipid
Function in cell-to-cell recognition and attachment
Viral entry mechanism → how virus gets into body/how we can stop viruses from entering body
Receptor proteins and viral entry
Most viruses use a glycoprotein called viral receptors to attach to a host cell
To get into body
HIV has gp 120 → looks for “glycoprotein 120” that adheres to human immune cells CD4 receptor
CD4: cell adhesion molecule that functions to keep other immune cells close by when immune response is generated
Same way find way in → same way to develop vaccines to keep them out
Lock and key mechanism
Asymmetric membrane
Plasma membranes are asymmetric as the inner surface differs from the outer surface
Interior is NOT identical to exterior
Examples:
Interior proteins anchor fibers of the cytoskeleton to the membrane
Inside cytoplasm anchor fibers to cytoskeleton
Exterior proteins bind to the extracellular matrix
Glycoproteins bind to the substances the cells need to import
Membranes have distinct inside and outside faces
Affects the movement of proteins synthesized in the endomembrane system
Endomembrane system
Group of membranes and organelles that work together to synthesize, modify, package, and transport proteins and lipids out using the:
Endoplasmic reticulum (ER)
Golgi apparatus
Nuclear envelope
Plasma membrane
Lysosomes
Ribosome → endoplasmic reticulum → golgi apparatus → golgi vesicle
Make protein → fold and modify protein → sort and package protein → transport protein to target destination
Membrane structure results in selective permeability
Works to keep things separated
A cell must exchange materials with its surroundings
A process controlled by the plasma membrane
Permeability of the lipid bilayer
Membranes are selective barriers
Smaller and nonpolar → easier to cross membrane
Size
Membrane is also nonpolar
Like dissolves like
Small, nonpolar molecules: can pass easily and quickly through and do not require proteins for transport
O2 and CO2
Small, polar molecules: more difficult than nonpolar; hydrophobic tails of the bilayer make it tougher and slower, but they can cross without the help of proteins
Ex: H2O
Large, nonpolar molecules: can pass through but it is also a slow process
Ex: carbon rings, glucose, nucleotides, amino acids
Large, polar molecules and ions: size and charge make it too difficult to pass through the nonpolar region of the phospholipid membrane without help
Ex: simple sugars and H+ ions
Transport across the plasma membrane
Cells must allow material to enter and exit
Permeability of the membrane allows cytosol (inside the cell) solutions to differ from extracellular (outside the cell) fluids (asymmetrical membrane)
Diffusion
Passive transport of any solute moving from areas of high concentration to areas of low concentration
Substances move down a concentration gradient
Ex: mix food coloring in water
Osmosis
Special type of diffusion!
Focus on the movement of water across a semipermeable membrane
Determined by solute concentration → things dissolved in the water
Water moves from areas of low solute concentration to areas with a higher solute concentration
Differences in water concentration occur when a solute cannot pass through the membrane
Adding more water into areas with a lot of solute
High blood pressure
reduce sodium intake
Salt sucks
More sodium → more blood volume increase → harder heart has to work
Water potential
Tendency of water to move from one place to another
Remember, water will move from areas of high solute concentration to areas of low solute concentration
Know Hypo and Hypertonic solutions
Animal cells → pressure required to stop the net movement of water
Hypertonic: cell can shrink
Hypotonic: cell can burst
Isotonic: ideal for animal cells
Plant cells → positive pressure caused by movement of water into a cell
Don't like isotonic situations
Prefer being in a hypotonic condition
Grocery store: spray water onto vegetables
Don't burst because they have a cell wall
Hypertonic situation: shrink
Tonicity
Ability of a solution to cause a cell to gain or lose water
Changes the volume in the cell by osmosis
Has great impact on cells without cell walls
Three different conditions
Isotonic
Hypotonic
Hypertonic
Animals and other organisms without rigid cell walls:
Live in hypertonic and hypotonic environments
Prefer isotonic
Isotonic solution
Concentration of solutes
Same inside and outside of the cells
Equilibrium
No net movement of water
Water flows both directions at an equal rate
Hypotonic solution
Concentration of solutes
Less outside in the solution than in the cell
Hypertonic cell
Cell will take in water
Hypo → under, beneath, less
Hypoglycemia → low blood glucose
Beneath normal level
Hypertonic solution
Concentration of solutes
Greater outside in the solution than in the cell
Cell will lose water
Hyper → over, excess, more
Hyperthyroid → too much thyroxine produced
Over the normal level
Normally put cell in salt water solution
Water balance of cells with walls (plants)
Cell walls: help maintain water balance
Plant cells are turgid/firm and generally healthiest in a hypotonic environment
Ideal situation
Osmoregulation by other organisms
Freshwater protists like paramecia and amoebas use contractile vacuoles which pumps water out of the cell to prevent bursting
Marine invertebrates have internal salt concentrations that match their environment
Fish excrete diluted urine which gets rid of excess H2O or salts
Osmoreceptors in the brain cells monitor solute concentrations in our blood
Releases hormones that affect kidney function
Passive transport → facilitated diffusion
No use of energy
Moves substances down their concentration gradients (high → low concentration)
Requires the use of transmembrane proteins:
Channel proteins
Carrier proteins
Span bilayer completely
Ions and large polar molecules use this for diffusion
Channel proteins
Transmembrane, completely spanning the membrane
Completely penetrate hydrophobic core of lipid bilayer
Some open all the time
Others are gated
Only open when a signal is received
Some only allow small molecules to pass through; larger molecules are too big to fit through the channel
Other channels are affected by charge
Attract positive ions; repel negative ions
Attract negative ions; repel positive ions
Only “appropriate” molecules pass into and out of the cell:
Size of molecule
Charge of molecule
Examples of channel proteins
Insulin receptor is a transmembrane receptor that is activated by insulin
Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis
Lock and key
Insulin receptor open → need to start taking glucose out of bloodstream and to other parts of body
Won't let other things sneak in
Scorpion venom contains chlorotoxin which can block channels in muscle cells that let chloride ions in and out
These ions normally help tell muscle cells when to relax
Paralyze victim
When channels get blocked, all the muscles flex at once leaving the animal tensed up and unable to move
Aquaporins are water channels
Transfer large quantities of water molecules across the hydrophobic plasma membrane
Kidney function and vision for animals
Water and nutrient movement; metal detoxification for plants
Carrier proteins (Passive)
Specific to a single substance
Binds to that substance
Changes shape
Carries it to the other side
Many allow movement in either direction
As concentration gradients change
Example: glucose transport proteins or “GLUTS”
Bloodstream to cells
All phyla of life have GLUTS
Very important
Main differences between Channel and Carrier Proteins
Protein channel
Open most of the time
Passage of molecules from outside in/inside out
Carrier proteins
More specific
Change conformation of protein molecule
Active transport across the plasma membrane
Must be against its concentration gradient (from low to high concentration)
Can also move ions against their electrochemical gradient
Like H+ ions to a solution that is more positive
Energy is ALWAYS required
Usually in the form of ATP (primary and bulk)
Atp powers process by shifting a phosphate group
From atp to the transport protein
Induces a conformational change of the protein
Then translocates the solute across the membrane
Energy source can also be an electrochemical gradient (secondary)
Produced as a product of primary active transport
Carrier proteins (Active)
Active transport can occur through transmembrane, integral carrier proteins called “pumps” and there are 3 types
Uniporters: carry 1 molecule or ion
Symporters: carry 2 different molecules or ions in the same direction
Antiporters: carry 3 different molecules or ions in different directions
Primary active transport
Moves an ion or molecule up/against its concentration gradient (from low to high)
Uses energy from atp hydrolysis
Always start with atp
Example: sodium-potassium pump
Moves 3 Na+ out of cell for every 2 K+ into cell using 1 atp
Primary example of active transport
Regulates nearly everything in out body
Maintain resting, heart beat, fire neurons, etc.
Hydrolysis of atp → adp allows for energy to transport ions across membrane
ATP hydrolysis “powers” secondary active transport
Electrochemical gradients: result from the combined effects of concentration and electrical gradients
Differential charges
Movement because moving from negative to positive/positive to negative
Movement caused by the gradient of ions across a membrane
Different charges across the membrane
Cytoplasm contains more negatively charged molecules (ions and proteins) than the extracellular fluid (fluid outside cell)
Electrogenic pumps: transport proteins, generates voltage across a membrane
Membrane potential → voltage difference across a membrane
Between in vs out
Important in the maintenance and functioning of our nervous system
Potassium chloride
Affects charge → stops the heart
Eliminates concentration gradient
Flooding heart with potassium
Secondary active transport
Moves an ion or molecule up/against its concentration gradient (low to high concentration)
Energy source not ATP→ electrochemical gradient
Many amino acids and glucose enter the cell in this way
Bulk transport
When cells need to import/export molecules/particles that are too large to pass through a transport protein
Large proteins or macro molecules
Endocytosis (importing)
Exocytosis (exporting)
Exocytosis
Outside of cellular process
Transport vesicles containing substances to be secreted fuse with the plasma membrane
Contents are then released outside of the cell into the extracellular fluid
Main function of the endomembrane system
Endocytosis
Into cellular process
Forming vesicles from plasma membrane and bring molecules INTO the cells for various functions
3 main methods
Phagocytosis
Eating
Solid particle encapsulated by forming vesicle
Pinocytosis
Fluid and aqueous particles
Cellular process of drinking
Ladle of soup into a bowl
Receptor mediated endocytosis
Very specific means of entry into cell
Receptor molecules that only take in substances that fit
Metabolism
Defined as the totality of an organism’s chemical reactions
Really complex
An organism’s metabolism:
Transforms matter and energy
Is subjected to the laws of physics
Amino acids take up space and have mass
Metabolic pathways
Consists of thousands of biochemical reactions that all require energy transformations
Many steps required and each step:
Is a separate chemical reaction
Catalyzed by a specific enzyme
End result is a “product”
Two types of pathways required to maintain the cell’s energy balance:
Catabolic
Anabolic
Example of metabolic pathway
Tyrosine is an AA that undergoes a series of reactions to make adrenaline (epinephrine) as a product
Fight or flight
Along the way, dopamine is synthesized at an intermediate step
Catabolic pathways
catabolism
Break down complex molecules into simpler ones
Ex: why you eat food
Release energy
Best example in biology: cellular respiration, the breakdown of glucose molecules
Large molecules are broken down into smaller ones → energy is released
Catabolic
Process: breaks down molecules
Polymer → monomer
Energy: released; exergonic
Delta G: negative
Spontaneous
Stability: more stable
Example: respiration; hydrolysis of ATP to ADP
Anabolic pathways
Build more complex molecules
Require energy
Top example in biology is photosynthesis
Small molecules are assembled into large ones → energy is required
Example: photosynthesis
Anabolic
Builds up molecules
Monomer → polymer
Energy: required; endergonic
Delta G: positive; non-spontaneous
Stability: less stable
Example: photosynthesis; synthesis of ATP to ADP
Energy
Capacity to cause change
Exist to perform work
Exists in various
Fundamental to all metabolic processes
Rearrange matter form one form to another
Sustains most of earth’s life
Comes from the sun
Bioenergetics
Study of energy flow through a living system
Types of energy
Potential energy: stored energy, the energy matter has because of its structure
Membrane potential
Na+ moving in/out of cell
Chemical energy stored in molecular structures
Like in glucose molecules
In a compressed spring
Can use if change is made to thing holding energy
Kinetic energy: energy in motion, movement of objects
Thermal energy: associated with the random movement of atoms or molecules
Heat: when thermal energy is transferred from one molecule to another
Energy can be converted from one form to another
Additional energy types
Free energy is similar to potential energy in that it describes the energy available to do work
Usable energy
A living system’s free energy: energy that can do work under cellular conditions
Gibbs free energy (G)
The free energy change of a reaction
Determines whether a reaction is spontaneous or nonspontaneous
G is affected by all chemical reactions/biological processes
After a reaction the change is denoted as delta G
Free energy
During a spontaneous change
Addition of external energy is NOT required
Free energy decreases and the stability of a system increases
Delta G is negative (look at slide)
This happens when energy is released in a chemical reaction
Example: creation of diamonds
An exergonic/spontaneous reaction: energy is exiting the system
Proceeds with a net release of free energy (negative delta G)
Net release of free energy to take reactants and create products
An endergonic/nonspontaneous reaction: energy is entering the system
Reactions that absorbs free energy from its surroundings
Delta G is positive
Smaller reactants/less free energy → take energy into system → make products excess of what we started out with
Activation energy
Initial energy required for a reaction to proceed/start
Heat energy from the surroundings is the main source in the cell
Usually obtained from the surroundings of the system
Helps reactants reach their transition state
Causes reactions to become contorted and unstable
Allows bonds to be broken or made
Once in this state, the reaction occurs very quickly
Enzymes function by lowering the Ea barrier, delta G is unaffected
Lowers activation energy
Reduce energy required to do reaction
Don't necessarily start the reaction; make reaction easier to begin
Thermodynamics
Study of energy transformations
The term system indicates the matter under study and the surroundings are everything outside the system
A closed system is isolated from its surroundings
Reactions in a closed system eventually reach equilibrium where delta G=0
Can’t put things in; can't take things out
Ex: saucepan with lid
An open system energy and matter can be transferred between the system and surroundings
Organisms are open systems
Constant flow of food in and waste out
Prevents equilibrium → G never = 0
Ex: saucepan without lid
Closed hydroelectric system
Water flowing downhill turns turbine that drives a generator providing electricity to a light bulb, but only until the system reaches equilibrium
Then becomes equal: turbine will not spin and light bulb will not work
Open hydroelectric system
Constant flow of water into tank
Opening out of the tank
Constantly putting water in and rushing water out
Constantly spinning turbine
Laws of thermodynamics
1st law of thermodynamics
Energy can NOT be created or destroyed
Energy CAN be transferred and transformed
2nd law of thermodynamics
Spontaneous changes (do not require outside energy) increase the entropy/disorder of the universe
Why organisms can’t simply recycle energy
Biological order and disorder
Biological systems are complex and highly ordered
Entropy: chaos/disorder
Entropy at the molecular level (overall trends, there are exceptions):
Entropy of a liquid state is greater than entropy of a solid state
Entropy increases when a substance is broken down into parts
Entropy increases as temperature increases
Entropy increases in reactions where the number of product molecules is greater than the number of reactant molecules
According to the second law of thermodynamics, entropy increases whenever something happens
Entropy → dispersal of energy
Natural tendency of a system and humans is for entropy to increase
How is order created and maintained in biological systems despite ever increasing entropy (disorder)?
Energy
Cells use a lot of energy to create and maintain order
Second law still holds true, entropy in the surroundings increases, largely due to the release of heat (also energy)
Internal cell maintain energy because of the use of energy
ATP: Adenosine Triphosphate
Cell’s primary energy shuttle
Energy $$
Powers cellular work by coupling exergonic and endergonic reactions
Back and forth changing
Chemical, mechanical, and transport work
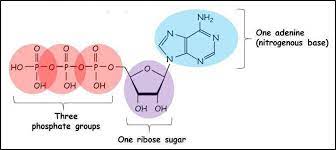
ATP Structure
Composed of an adenosine backbone + 3 phosphate groups attached
Adenine attached to ribose with phosphate tail
Tail has 3 phosphate (tri=3)
Broken down to ADP, accompanied by the release of energy
Break connection between two phosphates
Three phosphate → two
Adenosine diphosphate (ADP)
ATP hydrolysis: phosphorylation
Energy is released from ATP when a phosphate bond is broken
Anytime we do something to a phosphate group
ATP drives endergonic reactions by phosphorylation, transferring a phosphate to other molecules
ATP hydrolysis is reversible
Example: Sodium potassium pump
Main source of active transport
Using ATP and phosphorylation with ADP
Think of it as a battery
Energy coupling
Energy coupling: transfer of energy from catabolism to anabolism
Or the transfer of energy from exergonic processes to endergonic processes
Shifting back and forth between point A and point B
Example: photosynthesis and cellular respiration
Charged battery → hydrolysis reaction has energy released into anabolic/endergonic reactions
Energy-consuming processes
Dead battery → take single organic phosphate and undergo another organic reaction
Remove water molecule: dehydration synthesis; reattaching inorganic phosphate back to ADP and regenerate ATP molecule
Energy is form of catabolic/exergonic fractions
Energy releasing process
Spend money to earn money
Both processes → process of phosphorylation
Messing around with phosphate group
Adding or removing it
Enzymes speed up metabolic reactions by lowering energy barriers
A catalyst
An agent that speeds up a reaction without being consumed or permanently changed
Work efficiently and never be used up
If conditions allow it → varies with each enzyme
In body: body temperature (98 F), pH (6-8)
An enzyme → catalytic protein
Most end in ‘ase’
amylASE: helps change starches into sugars; found in saliva
maltASE: also found in saliva; breaks the sugar maltose into glucose; found in potatoes, pasta, and beer
helicASE: unravels DNA
Enzymes
Catalytic protein: agents that speed up reactions without being consumed or permanently changed
Do this by lowering the required activation energy
Very specific
Will only catalyze a single, specific reaction
Only binds to a specific reactant
Promotes bond-breaking and forming process
Lowering what takes to make the process started
4000 known biochemical reactions
Commercial uses include:
Synthesis of antibiotics
Detergents
Meat tenderizers
Brewing beer, making cheese, baking bread
Used outside body to help with everyday life
Activation barrier
Every chemical reaction requires the making and breaking of bonds
Activation barrier: energetic “hurdle” that a reaction must get over
What it takes for a reaction to occur
Some reactions have higher hurdles and some have lower hurdles
Low activation barrier allows a reaction to happen quickly
Enzymes lower energy barrier
Enzyme lifecycle
3D shape of the enzyme + the 3D shape of the substrate = specificity
Substrate (reactant) moves toward the enzyme’s active site
Chemical reaction is triggered by the enzyme
Enzyme releases the products
BE FAMILIAR WITH LIFE CYCLE AND LABELING PARTS
Enzyme activity
An enzyme’s activity can be affected by several factors:
Environment
Temperature: each enzyme has an optimal temperature
pH
Molecular components
Cofactors and regulatory molecules
Local conditions
Different tissues within the body
How much of enzymes should be produced
When enzymes should be produced
Where in cell enzymes should be produced
Different areas of the cell
Between different organisms
Enzyme activity: environment
Temperature: each enzyme has an optimal temperature at which they function appropriately
Almost every human enzyme → 96 F
Optimal temperature for enzyme of thermophilic → 75 C; 200 F
pH: each enzyme has an optimal pH at which they function appropriately
Humans → neutrality; around 7
Pepsin; stomach enzyme → very acidic
When things go “sub-optimal”: the denaturation of enzymes
Extreme conditions break the bonds that hold the folded structure
Denaturation: occurs when the structures of the proteins (enzymes) is disrupted due to unfolding
Denaturation makes the enzyme inactive, and this process is irreversible
Enzyme regulation
A cell’s metabolic pathways must be tightly regulated
To balance the catabolic and anabolic pathways and suit the cell’s current needs
For example: digestive cells in stomach work harder after a meal than when you are asleep
Knows what you need when you need it very accurately
Three primary ways to regulate enzymes:
When an enzyme should be active
Where an enzyme should be active
How much activity is needed
Regulation can be positive or negative!
Turn on/off
Very important
Both equally important
Enzyme activity: molecular components
Cofactors: non-protein enzyme components such as metals
Ex: NAD+ and NADP+
Coenzymes are organic cofactors including vitamins
Regulatory molecules: extremely important!
Positive or negative regulation
Inhibition: negative
Activation: positive
Allosteric: negative or positive
Concentration of these molecules is key
Enzyme inhibition
Competitive inhibitors
Similar shape to the substrate
Competing for the active site
Noncompetitive inhibitors
Bind to the enzyme at a different location
Changes the function → causes a slower reaction rate
Not in active site but changes the shape of the active site
Inhibitors negatively affect an enzyme’s activity
Down regulates
Stops/slows down reaction
Negative correlation
Example: disulfiram is a competitive inhibitor of aldehyde oxidase
Causes build of acetaldehyde and nausea and vomiting
Used to treat alcoholism → stops the breakdown of alcohol
Cyanide is a compound that acts as a noncompetitive inhibitor
Inner membrane protein in the mitochondria
Allosteric regulation: the “other object”
Almost every situation with noncompetitive inhibitor, you can call it allosteric
Allosteric not necessarily noncompetitive
Have unique set of criteria
When protein function at one site is affected by the binding of a regulatory molecule at a separate site
Enzymes change shape when regulatory molecules bind to specific sites, affecting their function → can cause activation or inhibition
Make reaction go faster or completely stop it
Nearly all cases of noncompetitive are allosteric regulation
However, allosterically regulated enzymes have set of unique properties that set them apart
I. e. Cooperativity, feedback inhibition
Allosteric regulation: cooperativity
Protein function at one site affected by binding of a regulatory molecule at a separate site
Think polypeptides and proteins
Inhibitor anywhere → whole protein gets shut down
Allosteric regulation: feedback inhibition
Feedback inhibition
The end product of the metabolic pathway shuts down the pathway
Usually at an early step by inhibiting an upstream enzyme
Important regulatory mechanism in cells
Example: ATP is an allosteric inhibitor for some enzymes involved in cellular respiration
Enzyme activity: local conditions
Within the cell, enzymes may be
Grouped into complexes
Incorporated into membranes
Contained inside organelles
Otherwise, found in different parts → different functionality
Energy transformations
Sun is the source of energy for almost all organisms
Solar energy is converted into a usable form that all organisms can use
Can indirectly use sunlight
Energy also leaves the system as heat
Energy in sunlight; energy out heat
Ecosystem
Respiration overview
Living cells require transformations of energy from outside sources to perform their many tasks
Cells extract energy from food
Generate ATP through the metabolic pathways involved that there is a close link between:
Transfer of energy
Movement of electrons
Which have high energy levels
Think of it as train station
Each process=train stop
Go from station to station until final destination
Cellular respiration (general)
Most prevalent and efficient catabolic pathway
Consumes oxygen and organic molecules like glucose
Yields ATP (its purpose)
Regeneration of ATP keeps cell working
Similar to combustion of vehicle
Engine; cell
Reaction: combustion; respiration
Transforming energy
Fuel: gasoline; eating food, oxygen
Power:
Move vehicle; work
Output:
Waste products: carbon dioxide and water
Redox reactions
Oxidation-reduction reactions
Oxidation: loss of electrons during a reaction
Reduction: gain of electrons during a reaction
They take place simultaneously
OILRIG
Oxidation is loss, reduction is gain
Reducing agent → oxidation reaction → oxidized
Reducing agent: glucose
Oxidized: carbon dioxide
Oxidizing agent → reduction reaction → reduced
Oxidizing agent: oxygen
Reduced: water
During cellular respiration (through series of enzymatic reactions)
Glucose is oxidized → produces carbon dioxide
Oxygen is reduced → produces water
Other important coenzymes:
FAD+; FADH
NADP+; NADPH
Coenzymes
non-protein molecules required for some enzymes to function
Act as electron carriers/shuttles
Other important coenzymes:
FAD+; FADH
NADP+; NADPH
Examples of Redox Reactions
Example: nicotinamide adenine dinucleotide (NAD)
Cofactor central to metabolism
Exists in two forms:
NAD+: oxidized form (fewest electrons)
Becomes reduced to form NADH
Accepts electrons during redox reactions
Least amount of negative charge → +
NADH: reduced form (most electrons)
Becomes oxidized to form NAD+
Donates electrons during redox reactions
Abundance of electrons
Cellular respiration (specific)
Energy-releasing chemical breakdown of fuel molecules
Chemical energy of organic molecules is released to make ATP
Provides energy for the cell to do work
Prokaryotes do NOT have mitochondria
Still do respiration → don’t do it in the mitochondria
Aerobic respiration
Energy needed for respiration is provided by the oxidation of glucose through a series of enzymatic reactions (steps)
To pass electrons to their carriers, usually NAD+ first
Utilizes NAD+ and the electron transport chain
Goal is to produce ATP
Not a single step → would be explosive
If one step, there would be explosions when you eat
A large release of energy (stored in bonds)... similar to the reaction of hydrogen and oxygen to form water
Want to slowly generate ATP
ATP in living systems
ATP generation
By endergonic reactions
ADP undergoes phosphorylation
ADP + P → ATP
Releasing energy to do work
Energy required can be obtained from
Substrate level phosphorylation (inefficient during cellular respiration)
Direct transfer for a phosphate group
Coupled exergonic/endergonic reactions during the breakdown of glucose
Chemical energy
Occurs during glycolysis and Krebs cycle (inefficient)
Oxidative phosphorylation (chemiosmosis and electron transport chain - VERY efficient)
Process that requires extra steps and ATP synthase
90% ATP produced by this method (very efficient)
Occurs in the mt (mitochondria), cp (chloroplast), and membrane of aerobic prokaryotes
Uses energy from a proton gradient generated during ETC (electron transport chain)
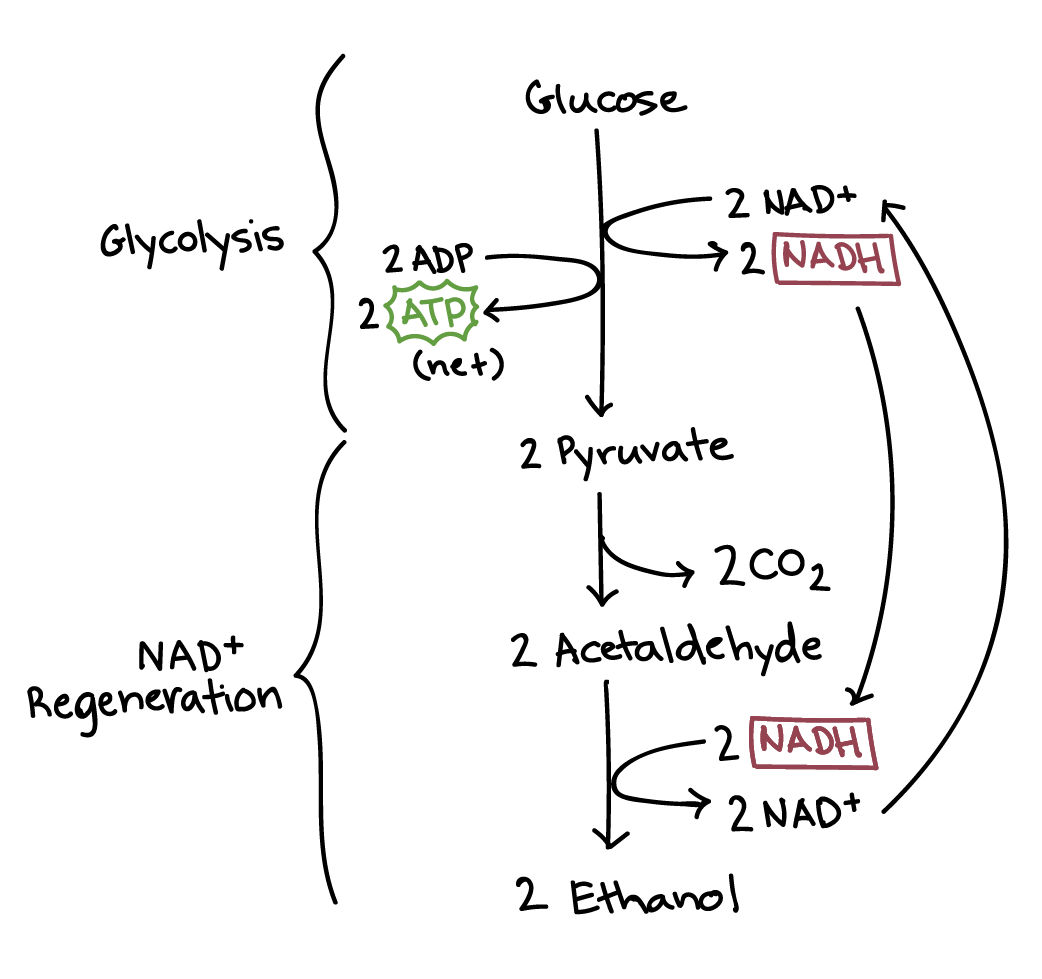
Glycolysis
Doesn’t occur in the mitochondria
Glycolysis: universal energy releasing pathway
Greek: splitting of sugar
First step in oxidation of glucose
Occurs in the cell cytoplasm
Everything has cytoplasm rather than mitochondria
Oxygen not required
Inputs
1 glucose
2 NAD+
2 ATP
Products
2 ATP (NET)
2 NADH and 2 H+
2 pyruvate
Glucose goes in; pyruvate goes out
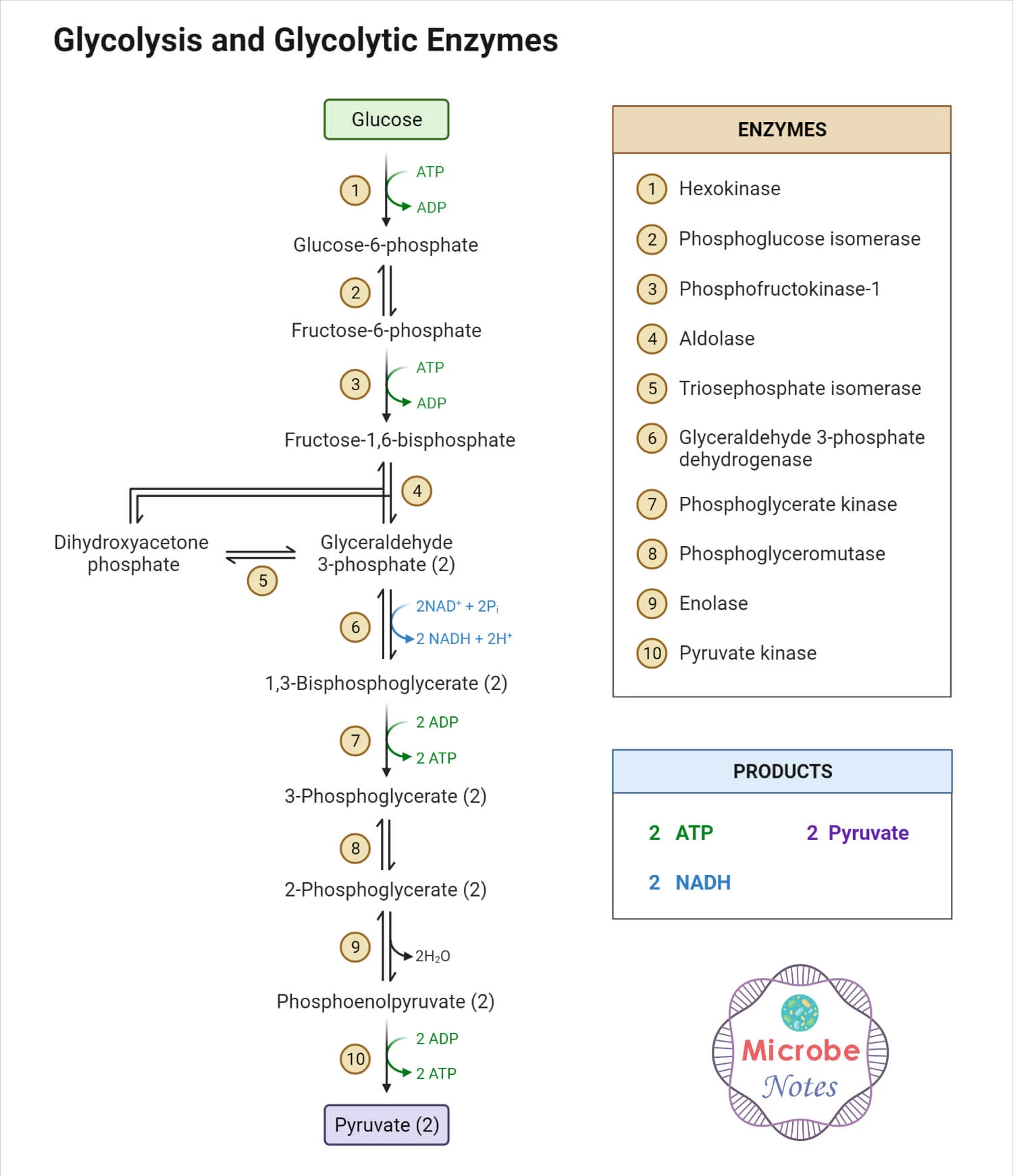
Glycolysis: energy investment
First 5 steps → energy investment stage
Take glucose and use energy to transform the glucose
“Spending money”
Have to spend money to earn money
Use energy to convert glucose of pyruvate
Kinase: enzymes that transfer phosphates (groups)
Isomerase: enzymes that catalyze reactions involving structural rearrangements
Forms isomers
Same molecular formula but different arrangements
Stage 1-2: use up 2 ATP
Stage 3-4: use up another ATP molecule
Why is energy invested?
Reaction becomes more ordered/organized
Start with glucose (1 molecule) → series of changes → use up energy → more ordered system → G3P (know have 2 molecules)
G3P: transition molecule/intermediate molecule
Alert that we went from investment phase to payoff phase
Glycolysis: energy payoff
Happens twice → each molecule of G3P
Undergoes last 5 stages of glycolysis
Payoff phase yields:
2 NADH → moved to ETC in mitochondria
2 H+ (proton; hydrogen ion)
4 ATP → can be used in cytoplasm for anabolic processes
2 H2O
2 Pyruvate → enters mitochondria, broken down in pyruvate oxidation and citric acid cycle
One cycle of glycolysis makes 2 ATP
Only “net” 2 ATP
Eventually produces 4 but uses 2 ATP to make 4
Spend money to make money
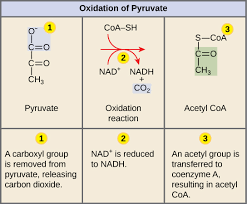
Pyruvate oxidation
Pyruvate is transported to the matrix of the mitochondria of eukaryotes
Prokaryotes do this in cytoplasm
Remember: 2 pyruvate per glucose
Pyruvate cannot enter the citric acid cycle (CAC) unless it is altered
Process produces carbon dioxide whenever a carbon is removed
Titilizes coenzyme A (CoA)
Carrier compound that picks up, activates, and transports the transformed pyruvate
Results in acetyl CoA
Major function is to deliver the acetyl group to the next stage of glucose catabolism
Input: 2 pyruvate
Output: carbon dioxide, acetyl CoA
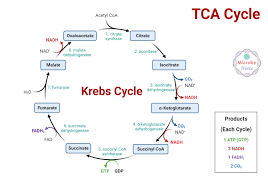
Citric Acid Cycle (Krebs Cycle)
8 enzymatic steps
Redox
Dehydration
Hydration
Decarboxylation
Occurs in the matrix of the mitochondria (double membrane through endosymbiosis)
Prokaryotes accomplish in their cytosol
Does NOT directly consume oxygen
But DOES require it
Produces very little ATP
Purpose is to gather electrons from the ETC
Completes the energy-yielding oxidation of organic molecules
Every turn of cycle: more electrons gathered
Because of oxidation of organic molecules
Harvesting electrons from enzymatic reactions
Inputs: NO pyruvate; 2 acetyl CoA, 2 oxaloacetate, 3 NAD+, 1 FAD
Outputs: 4 CO2, 3 NADH, 1 FADH, 2 ATP/GTP, H2O
Oxidative phosphorylation
Occurs in the cristae (inner membrane)
Happens in cell membrane of prokaryotes
More surface area to perform electron transport chain
Efficient ATP production
A LOT of ATP production (the most)
Bulk majority of energy
Only pathway where O2 is an input
Consists of:
Electron transport chain
Creates a H+ concentration gradient
Provides the energy to power chemiosmosis
Chemiosmosis
Generates ATP
Couples the process of electron transport to ATP synthesis
Follow flow of electrons
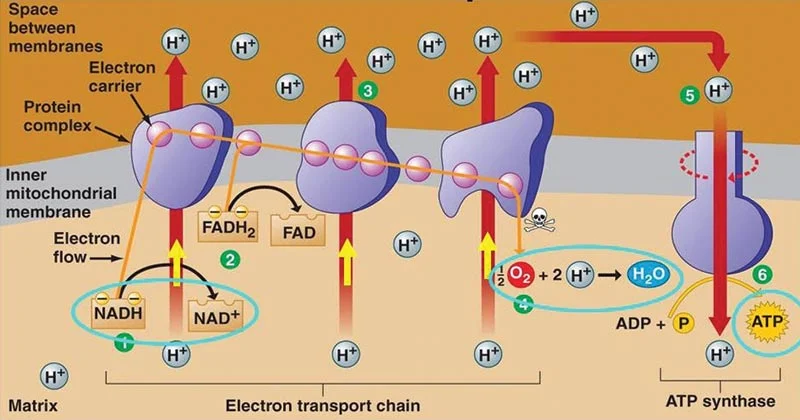
Electron transport chain
Electrons are passed from one component of the ETC to the next
Via a series of electron transporters embedded in the inner mitochondria membrane
Shuttle electrons from NADH and FADH2 to O2
Electron flow is unidirectional → only go one way
Oxygen is the final electron acceptor at the end of the ETC and creates water → reason why we breathe
Complex 4 get to oxygen → water
End of station/line for electron movement
Causing protons to be pumped out at each station
Gathering protons
Protons in matrix and membrane very different
In the process…
Protons (H+) are pumped from the matrix to the intermembrane space
O2 is reduced to form H2O by gaining electrons from the ETC
Protons are returned to the matrix but must pass through ATP synthase which then is activated and adds a phosphate to ADP, making ATP again
Chemiosmosis
Energy coupling mechanism
Uses kinetic energy to form ATP from ADP+Pi
Through the energy generated from protons (H+) falling down its gradient (electrochemical gradient)
ATP synthase
Integral protein (enzyme)
Bottom left gif
Phosphorylating as each proton passes through
Catalyzes the assembly of ATP
H+ gradient
Stores energy
Referred to as a proton-motive force
Drives chemiosmosis in ATP synthase
Anaerobic Metabolism
Absence of oxygen
Fermentation takes place in the absence of oxygen
Fermentation → a type of anaerobic respiration
Catabolic process → partial degradation of sugars
Occurs in cytoplasm of ALL organisms
Both prokaryotes and eukaryotes
Enables some cells to produce small amounts of ATP
Without oxygen
Sometimes when organisms need it the most
Consists of:
Glycolysis: universal pathway
NAD+ regeneration pathway
This varies based on the type of fermentation
2 types:
Lactic acid fermentation
Alcohol fermentation
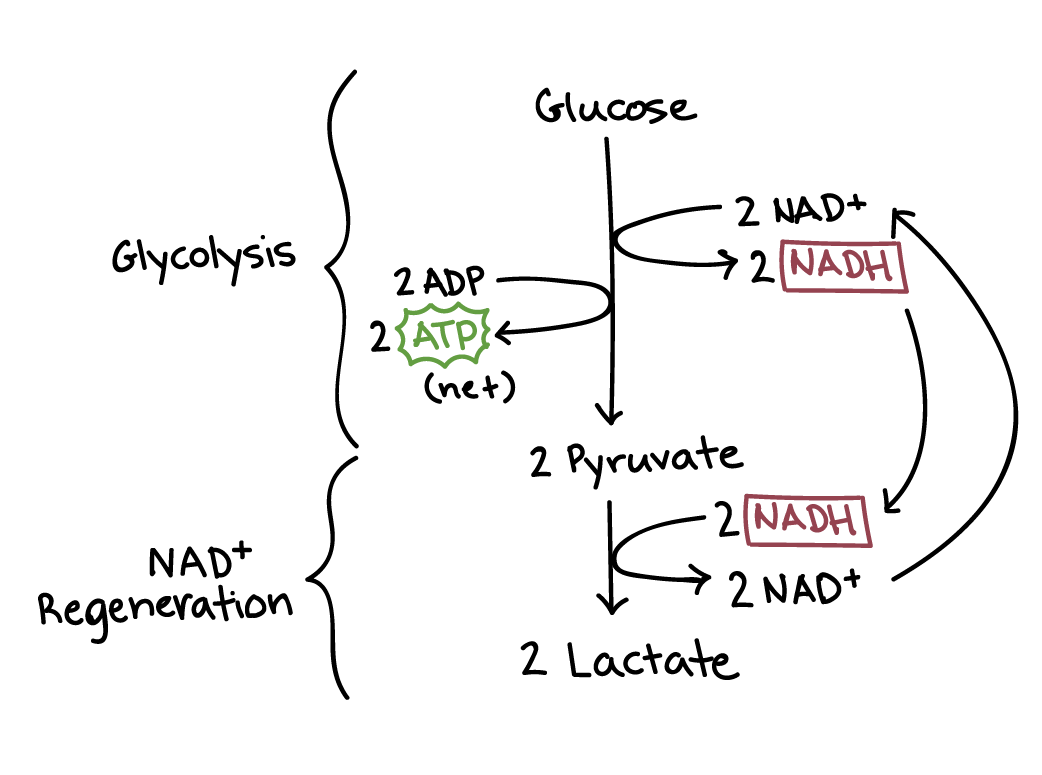
Lactic acid fermentation
Occurs in many organisms
From bacteria to humans
Pyruvate is converted to lactate
Directly regenerates NAD+
NADH is oxidized to NAD+
No CO2 released here
As featured in
Muscle cells
When O2 is limited
Mammalian red blood cells
No mitochondria
Some bacteria
Like the ones seen in yogurt
Pyruvate = final electron acceptor
Alcohol fermentation
Occurs mainly in yeasts (esp. Anaerobic species)
Pyruvate is converted to ethanol (EtOH)
2 steps/reactions
Releases O2
Pyruvate → acetaldehyde → EtOH (alcohol)
Releases carbon dioxide
Happens from transition of pyruvate to acetaldehyde
Acetaldehyde is the final electron acceptor
Regulation
Cells strive for efficiency
This is why, if we eat more food (chemical energy) than we need, our bodies will convert it to a storage molecule (like fat or glycogen)
Excess amino acids result in the anabolic pathway in protein metabolism to be turned off/slowed down
Common mechanism: feedback inhibition
Heterotrophs
“Other feeding” organisms
Obtain their organic material from other organisms
Are the consumers of the biosphere
Anything above primary producer
Autotrophs
“Self-feeding” organisms
There are two types:
Photoautotrophs: use sunlight to make food
Plants, algae, and cyanobacteria
Chemoautotrophs: energy from inorganic compounds (chemicals) to make food
Thermophilic bacteria that live in a thermophilic environment
Locating photosynthesis
Chloroplasts: major site of photosynthesis
Most abundant in leaves
Basically anywhere that is green
Highest density of chloroplasts in the mesophyll cells
Chloroplast structure:
Double membrane: inner and outer
Same reason as mitochondria
Endosymbiotic origins
Stroma: inner space of the chloroplast
Contains the thylakoids/grana
Goo
Site of dark reactions
NOT STOMA → pores in leaves
Thylakoid: disk shaped structure containing chlorophyll
Site of light reactions
Grana: stacks of thylakoids
Lumen: inner space of the thylakoid
Tracking atoms through photosynthesis
Just like cellular respiration:
Involves a series of complex metabolic pathways
Photochemical reactions
“Light reactions”
“Light dependent reactions”
Happens in presence of light
Biochemical reactions
“Dark reactions”
“Light independent reactions”
Calvin cycle
Photosynthetic reactants
Before exploring the overall process of photosynthesis, let’s establish the sources of its components:
Water is absorbed by the roots of the soil
Carbon dioxide is acquired from the air as a result of gas exchange through the stomata
Singular = stoma
Sunlight (energy from sun) is absorbed in chlorophyll
The use of water in Photosynthesis
Chloroplasts split water into hydrogen ions, oxygen, and electrons
Incorporates the electrons of hydrogen into sugar molecules
Requires a LOT of energy as an input and releases oxygen as a byproduct
Oxygen is a waste product
Redox reaction:
Water is oxidized, carbon dioxide is reduced
Light energy
Form of electromagnetic energy (radiation)
Composed of photon particles that travel as waves
We can only see a fraction of this energy
Visible range of light energy (same range plants use)
Represented on the electromagnetic spectrum
The entire range of electromagnetic energy
Light reactions convert solar energy to chemical energy → ATP and NADPH
Wavelengths and the visible spectrum
Wavelengths are typically measured in nm
Longer wavelengths
Crest farther apart
Carry less energy than short wavelengths
Visible range (400-700 nm)
Violets have the shortest wavelengths
More energy
Reds have the longest wavelengths
Less energy
Light absorption
Pigments: substances that absorb specific light wavelengths (photon energy)
Each has a unique absorption spectrum
Photosynthetic pigments: set of pigments that absorb visible light and transfer the energy form the photons
Three main:
Chlorophyll a
Chlorophyll b
B-carotene
Absorption spectrum
Shows which wavelengths of light are being absorbed by a given pigment
Chloroplast pigments provide clues into which wavelengths are most effective for photosynthesis
Peak at blue/violet and red; low at green
Absorb well/terribly
Theodor engelmann: credited for demonstrating the action spectrum for photosynthesis
Measuring output of oxygen
Placed a filament of green algae in a light spectrum created by a prism
Exposed different segments of algae to different wavelengths
Put aerobic bacteria on the slide and observed where these grew best
Bacteria that requires oxygen to grow
Waited to see where bacteria would best grow → taking in oxygen and reproducing
Where there is oxygen → there is photosynthesis → where bacteria grows the most
Bacteria grew best in violet/blue and red sections
Comparing absorption vs action spectra
Where they absorb most they act out/release oxygen the most
Action spectrum resembles the absorption spectrum for chlorophyll a but does not match exactly
Close correlation
Rate of photosynthesis → oxygen output
Peaks at wavelengths that absorb the most light (violet/blue and red)