Chemotherapy Exam #1 (copy)
1/140
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
141 Terms
What is cancer?
a collection of diseases characterized by the abnormal growth and spread of mutant cells in the body
angiogenesis
The physiological process by which new blood vessels grow from pre-existing ones.
How does cancer kill?
(not fatal if confined within non-essential organs)
spread or metastasize
compromise essential organ functions
Non-essential organs
appendix
gallbladder
spleen
reproductive organs
Tissue - Epithelial → cancer type?
Carcinoma
Tissue - Connective Tissues → cancer type?
Sarcoma
Tissue - Blood-related cells and tissues → cancer type?
Leukemia, lymphoma, and myeloma
Tissue - Nervous system → cancer type?
Glioma, glioblastoma, neuroblastoma
Cancer type → Carcinoma
Skin, breast, lung, prostate, etc
Most common human cancer
Cancer type → Sarcoma
bones, cartilage, tendons and fibrous tissues
Cancer type → Leukemia, lymphoma, myeloma
blood marrow, lymphocytes, plasma cells
Cancer type → Glioma, glioblastoma, neuroblastoma
glial and immature nerve cells
What was chemotherapy originally described as?
The use of chemicals (drugs) that are selectively toxic to invading microorganisms
What was chemotherapy described as now?
describing drugs in cancer treatments
What does chemotherapy refer too?
the type of anticancer drugs that are generally toxic to rapidly-dividing/growing cells
What are the three types of cancer treatment?
surgery
radiation therapy
chemotherapy
Surgery
solid (non-hematological) cancers may be cured if entirely removed by surgery, but this is not always possible
Radiation Therapy
is the use of ionizing radiation to kill cancer cells and shrink tumors
Chemotherapy
is a cancer treatment with systemic application of drugs to kill or inhibit the growth of cancer cells
What can cancer treatments be used for?
cure cancer
control cancer
ease cancer symptoms
What does cure cancer refer to?
remission
complete remission for 5 years or more
Remission
the condition in which treatments have reduced the signs and symptoms of the cancer
can be partial or complete
control cancer
continually use of chemotherapy to keep cancer from growing/spreading or to slow the growth
ease cancer symptoms
shrinks tumors that are causing pain or pressure
Traditional Chemotherapy
target dividing cells (kill or inhibit the growth of cancer cells)
it can also harm normal cells that divide rapidly. Damage to normal cells may cause serious side effects.
Selectively target cancer cells while sparing normal cells in the body
understanding the differences between normal and cancer cells is fundamental to developing cancer therapeutics
Forms of anticancer agents
small molecules
biologics
Small molecules
chemicals
chemotherapy
targeted therapy
immunotherapy
Biologics
antibodies
Engineered immune cells
targeted therapy
immunotherapy
Common adverse effects of cancer drugs
immunosuppression
myelosuppression
anemia
gastrointestinal distress
nausea
vomiting
fatigue
hair loss
secondary malignancy
infertility
teratogenicity
death
What are serious adverse effects due to?
the lack of selectivity between normal and tumor cells
Majority of cells in human bodies are…
differentiated (they no longer grow)
differentiated cells…
acquired one or more DNA mutations that cause cells to re-enter into cell proliferation phase
Uncontrolled cell growth is…
one characteristic of cancer
cancer treatments
surgery
radiation therapy
chemotherapy
Surgery
solid (non-hematological) cancers may be cured if entirely removed by surgery, but this is not always possible
radiation therapy
is the use of ionizing radiation to kill cancer cells and shrink tumors
Chemotherapy
a cancer treatment with systemic application of drugs to kill or inhibit the growth of cancer cells
Definition of Chemotherapy
originally used to describe the use of chemicals (drugs) that are selectively toxic to invading microorganisms
more recently, frequently used for describing drugs in cancer treatments
refers to the type of anticancer drugs that are generally toxic to rapidly-dividing/ growing cells
Forms of anticancer agents
small molecules
biological agents
Small molecules
chemicals
used in
chemotherapy
targeted therapy
immunotherapy
Biological Agents
antibodies
nucleotides
engineered immune cells
used in
targeted therapy
immunotherapy
goal of chemotherapy
to inhibit cell proliferation and tumor multiplication, thereby avoiding cancer invasion and metastasis
3 main goals for chemotherapy in cancer treatment
cure
control
palliation
cancer cells depend on cell cycle to duplicate cancer DNA to pass on to daughter cells
Cells depend on the cell cycle to efficiently duplicate their genomes and undergo cell division
There are four phases of the cell cycle: G1, S, G2, and M
note G0 phase is when cells exit the cell cycle and enter quiescent phase
Traditional chemotherapeutic drugs are anticancer drugs that can affect all cell types (cancer and non-cancer)
work by killing or inhibiting growth of cancer cells due to the fast cancer cell proliferation
Anti-cancer drugs
Although different chemotherapeutic drugs have different mechanisms of action, they all either inhibit cell growth or induce cell death
Traditional chemotherapy agents primary interfere with DNA, RNA, or protein synthesis or affecting appropriate function of enzymes
Chemotherapeutic agents can either be specific or independent of cell cycle stage
4 major classes of chemotherapy agents
alkylating agents
antimetabolites
plants alkaloids
antitumor antibiotics
cell cycle specific drugs
more effective to cancers in which cells proliferate very fast
cell cycle-independent drugs
typically useful in both slow and fast growing cancers
mechanisms of chemotherapy drugs
dna
dna replication
mitosis
Mechanisms of chemotherapy drugs - DNA
alkylating agents
cyclophosphamide
ifosfamide
Platinum
cisplatin
carboplatin
oxaliplatin
DNA corsslinks
Mechanisms of chemotherapy drugs - DNA Replication
topoisomerase inhibitor
irinotecan
etoposide
antitumor antibiotics
doxorubicin
epirubicin
bleomycin
Antimetabolites
methotrexate
5-fluourourcil
gemcitabine
hydroxyurea
Blocking enzymes involved in DNA replicaiton
Mechanisms of chemotherapy drugs - Mitosis
Microtubule inhibitors
docetaxel
paclitaxel
vinblastine
vincristine
vinorelcine
Impairing the formation of spindle microtubules formation
Alkylating agents
Mechanism: alkylating agents directly damage DNA by
adding an alkyl group to the guanine residue in DNA (monoalkylated)
cross-linking two adjacent Guanine residues together
intra-strand cross-links
inter-strand cross-links
Inhibit DNA replication and transcription
Cell cycle independent agents
Examples of Alkylating Agents
Nitrogen mustard - bendamustine, cyclophosphamide, ifosamide
Nitrosoureas - carmustine, lomustine
Platinum analogs - carboplatin, cisplatin, oxaliplatin
Triazenes - darcarbazine, procarbazine, temozolamide
Ethyleneimine - thiotepa
Alkyl sulfonate - busulfan
Clinical use of alkylating agents
leukemia
lymphoma
hodgkin disease
multiple myeloma
sarcome
lung
breast
ovarian
brain
Platinum coordination complexes - Mechanism
usually with alkylating agents as DNA-damaging agents because they also form intra- or inter-strand crosslinking
generally most effective at treating slow-growing cancers
Platinum coordination complexes - Examples
cisplatin (platinol): effective against testicular cancer
oxaliplatin (Eloxatin): often used in combination therapies for the treatment of advanced colorectal cancer
Carboplatin
Platinum coordination complexes - Clinical use (Cisplatin)
leukemia
lymphoma
breast cancer
testicular cancer
ovarian cancer
head and neck cancer
cervical cancer
sarcoma, cancer that starts in bone and soft tissue
Cytotoxic antibiotics
several antibiotics have potent antitumor activity (too toxic to use as antibiotics) and were developed as anticancer agents
They are grouped together, even though they act through different mechanisms and have widely different indications
Examples of Cytotoxic antibiotics
Belomycin (Blenoxane)
Dactinomycin (Cosmegen)
Cytotoxic antibiotics - Belomycin
mechanism: the exact one is unclear, but it involves an oxygen and metal-ion-mediated reaction. Overall, it induces single- and double-strand DNA breaks
Clinical use:
squamous cell carcinoma
melanoma
sarcoma
testicular cancer
Hodgkin’s & non-Hodgkin’s lymphoma
Cytotoxic antibiotics - Dactinomycin
Mechanism: intercalates into DNA (binds in between double-stranded DNA) and blocks the progression of transcription machinery. Does not usually generate DNA breaks
Topoisomerase (Topo) inhibitors
inhibits two different classes of enzymes
topo 1 (Class 1 enzyme)
topo 2 (class 2 enzyme)
Mechanism: binds to the enzymes on DNA and locks the enzyme into a covalent-lined protein DNA adduct. This is also known as topoisomerase poisoning. Enzyme-DNA adducts inhibits DNA replication, causing the DNA replication machinery to collapse, thereby generating DNA double-strand breaks
Examples of Topo inhibitors
etoposide (plant alkaloid)
doxorubicin (anthracycline antibiotic)
Topoisomerase 1 poison - Camptothecin
inhibits Topo 1 enzyme at the religation step of the single stranded DNA by covalently bind enzyme to DNA adduct
Topoisomerase 2 poison - Etoposide & doxorubicin
mechanism: they bind to human topoisomerase 2 (alpha and beta) on DNA and convert a topoisomerase 2 (an essential enzyme) into a covalently-linked protein adduct (a cellular poison) on the genome (topoisomerase poisoning)
DNA replication process
copy the DNA with high fidelity
incorporating nucleotides to complement pair A-T and C-G during DNA replication
dNTPs - N refers to any nucleotides (A, T, C, G or U)
Anti-metabolites - mechanism of action
interfere with DNA and RNA synthesis, disrupting the growth and division of rapidly proliferating cells, including cancer cells
maximal cytotoxic effect in S phase (thus considered as cell-cycle specific chemotherapeutic agent)
Anti-metabolites - types
antifolate antimetabolites
methotrexate
Anti-pyrimidine and anti-purine antimetabolites
5-fluorouracil
Nucleotide analogs
cytarabine
antifolate antimetabolite - methotrexate
undergoes a series of transformations and is eventually converted into a compound called methotrexate polyglutamate. This active form of methotrexate inhibits the enzyme dihydrofolate reductase (DHFA)
inhibits purine (dATP and dGTP) synthesis, causing imbalance dNTP pools, thereby pausing DNA replication and repair
anti-pyrimidine antimetabolite - 5-fluorouracil
5FU - analogue of Uracil with a Fluorine atom at C-5 position instead of a hydrogen
5FU converts to 3 main active metabolites
fluorodeoxyuridine monophosphate (fdUMP) - major metabolite
fluorodeoxyuridine triphosphate (FdUTP)
fluorouridine triphosphate (FUTP)
FUTP disrupts RNA synthesis
FdUMP inhibits Thymidine synthase, causing imbalance in dNTP pool, thereby perturbing DNA replication & repair
Nucleotide analogues - cytarabine
instead of developing inhibitors targeting enzymes in dNTP production, we can also develop nucleotide analogues to mimic the natural dNTP molecule
converts from Ara-cytosine to Ara-CTP (CTP analogue)
Blocks DNA polymerase from synthesizing new DNA strand
What is crucial for separating chromosomes in mitosis?
Microtubules
Microtubules polymerization/depolarization is…
GTP hydrolysis dependent
Microtubules inhibitors
Vinca alkaloid - assembly inhibition (polymerization)
Taxanes - disassembly inhibition (depolymerization)
Vinca Alkaloid
Vincristine
Vinblastine
binds to tubulin dimers, inhibiting assembly of microtubules structures → arresting mitosis in metaphase
Taxanes
Paclitaxel
stabilizes microtubule polymers and blocks their disassembly → blocks the progression of mitosis
prolonged cells in mitosis by activating mitotic checkpoint triggers apoptosis
Pharmacological Approaches to Cancer Treatment
Understanding the differences between normal and cancer cells is fundamental to developing cancer therapeutics
Selectivity
Specificity
eliminate cancer cells or suppress cancer cell growth/speed
Selectivity
targeting cancer cells while sparing normal calls
specificity
affecting a specific molecular target
Selectivity (Cancer vs Normal Cells)
proliferating (rapid) vs non-proliferating (slow)
Alkylating agents (platinum)
crosslinking DNA
Cell cycle-indep
Antimetabolites (5-FU)
DNA synthesis
cell cycle specific
Microtubule inhibitors (Taxanes)
mitosis
cell cycle specific (M-phase)
fundamental features underlying cancer growth and metastasis
sustaining proliferative signaling
evading growth suppressors
enabling replication immortality
activating invasion and metastasis
inducing angiogenesis
resisting cell death
Aberrant growth and Metastasis
aberrant growth
Proliferate
sustaining proliferative signaling
enabling replication immortality
evading growth suppressors
Survive
Resisting Cell Death
Size increase
inducing angiogenesis
Metastasis
activating invasion and metastasis
Sustaining proliferative signaling - Cell proliferation signals
extracellular signals instruct cells to proliferate
growth signals - growth factors, cytokines, hormones
Receptors
cell surface receptors (ex: growth factor receptors)
Nuclear receptors (ex: hormone receptors)
Transduce signals to promote cell growth
Sustaining proliferative signaling - epidermal Growth Factor (EGF) signaling
An example of growth factor signaling
Growth factors
extracellular proteins
EGF family
Receptors
receptor tyrosine kinases
EGFR family (EGFR, HER2, HER3, HER4)
Signal transduction pathways
cascades of protein phosphorylation
MEK/ERK pathway
PI3K/AKT pathway
Deregulation of Growth Signaling in Cancers - Genetic mutations that deregulate growth signaling
gain-of-function mutations
oncogene
a gene that drives cancer development
viral gene:v-RAS, mutated cellular gene RasV12
proto-oncogene
a gene with normal cellular functions that becomes an oncogene due to mutations
RAS, PI3K, EGFR
Loss-of-function mutations
tumor suppressor
a gene that normally suppresses cancer development
PTEN
Sustaining proliferative signaling - therapeutic intervention
For deregulated EGFR signaling activation
EGFR inhibitors
MEK/ERK signaling
PI3K/AKT signaling inhibitors
Evading growth suppressors - Cell cycle control
a tightly regulated process through which cells divide
G1 → S → G2 → M
Cell cycle machineries
positive, regulators:
cyclins, cyclin-dependent kinases (CDK) (proto-oncogenes)
Negative regulators
cyclin-dependent kinase inhibitors (CDKI) (tumor suppressors)
Cell cycle checkpoints
DNA replication defects
DNA damage
chromosome spindle attachment defects
Evading growth suppressors - Defective cell cycle control in cancers
mutation or deregulated expression of cell cycle machineries
CyclinD, CDK6
Mutation or loss of cell cycle checkpoint regulators
p53
Note:
p53 is a major tumor suppressor
later studies found that gain-of-function p53 can drive cancer growth (oncogene)
Evading growth suppressors - Therapeutic intervention
CDK4/6 inhibitors
Enabling Replication Immortality - Cell Replication Limit
Hayflick limit
a finite number of divisions before normal cells stop replicating and senesce (irreversible growth-arrest)
Replicative Senescence
a cellular mechanism that prevents unlimited cell replication
Enabling Replication Immortality - Chromosome end replication
telomere
a repetitive DNA sequence at chromosome ends
allows replication of the chromosome ends
protects chromosomes from deteriorating or fusing
critically short telomeres trigger cellular senescence
Telomerase
an RNA-dependent DNA polymerase
adds telomere sequences to chromosome ends to maintain telomere length
low level in normal somatic cells
higher levels in stem cells
Enabling Replication Immortality - Deregulated telomerase expression in cancers
elevated telomerase activities in many cancer
Enabling replication immortality - Therapeutic intervention
oligonucleotide telomerase inhibitors (approved in june 2024)
Resisting cell death - cell death induction
intrinsic trigger
genome damage
lack of survival signal
activation of oncogenic signals
Extrinsic trigger
tumor necrosis factor (TNF) signaling
Resisting cell death - Apoptosis
programmed cell death
regulated by the Bcl-2 protein family
anti-apoptotic: Bcl-2, Bcl-xL, Mcl1
pro-apoptotic: Bim, Bax, Bid, Bad
mediated by a cascade of Caspase-dependent events
caspase is a protein family of cysteine proteases
Resisting cell death - Deregulated Apoptosis in Cancers
cancer cells can deregulate apoptosis by altering:
pro-apoptotic and anti-apoptotic protein levels
pro-apoptotic and anti-apoptotic protein phosphorylation (regulates functions)
caspase expression
Resisting cell death - therapeutic intervention
inhibitors of anti-apoptotic proteins
ex: Bcl-2 inhibitors
!note: other types of programmed cell death are also involved in cancer!
necroptosis
ferroptosis
Inducing angiogenesis - Angiogenesis
a process of sprouting new blood vessels from existing ones
Tumors cannot grow beyond a certain size without blood vessels to supply oxygen and nutrients
Induced by vascular endothelial growth factor (VEGF) signaling
Inducing angiogenesis - Therapeutic intervention
VEGF inhibitors
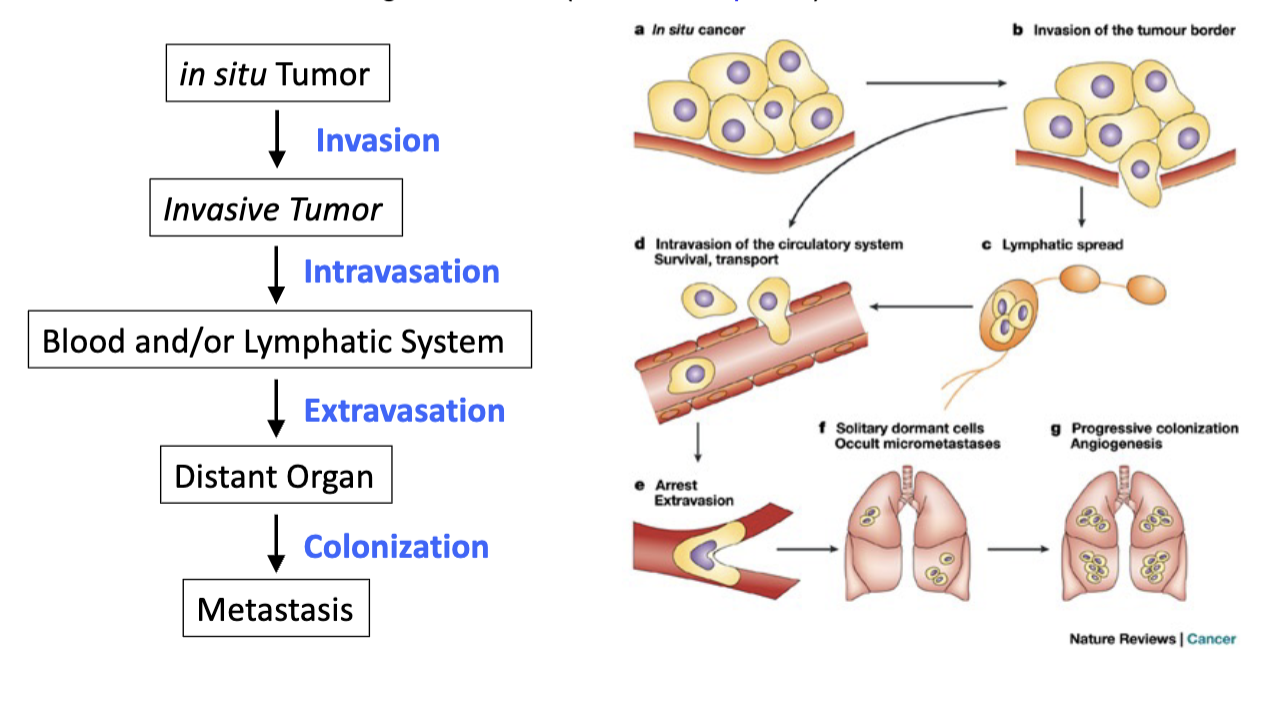
Activating Invasion and Metastasis
tumor cells spread and grow in distant organs - metastasis
advanced stages of cancer