ME215 Exam 2
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
108 Terms
Heat Treatment
processes of controlled heating and cooling to purposefully alter a material's structure and properties
Full Annealing - Hypoeutectoid
Time and energy consuming Process of heating and slowly cooling material to remove internal stresses and toughen it, resulting in coarse pearlite and excess ferrite, resulting in soft and ductile steel
Full Annealing - Hypereutectoid
Time and energy consuming Process of heating and slowly cooling material to remove internal stresses and toughen it, resulting in coarse pearlite and excess cementite
Normalizing
Process of heating steel to temperature higher than annealing and then cooling with uncontrolled air. More cost effective than annealing and properties will vary between surface and interior due to different cooling rates
Process Anneal
Recrystallization is induced after a material has been cold worked to reduce strain hardening effects. Induces a change in grain size, shape, and distribution
Stress-relief anneal
Reduces residual stresses in casting, welded assemblies, and cold-formed products, slow cooled
Spheroidization
Objective is to produce a structure in which all of the cementite is in the form of small spheroids or globules dispersed throughout a ferrite matrix
Solid-solution strengthening
Base metal dissolves other atoms as substitutional solutions or interstitial solutions
Strain hardening
Increases strength by plastic deformation
Grain size refinement
Metals with smaller grains tend to be stronger
Precipitation hardening ( or age hardening)
Strength is obtained from a nonequilibrium structure produced from heat treatment. most effective mechanism to strengthen nonferrous metals. heating, quenching, aging
Dispersion hardening
When two or more phases exist, Dispersing second-phase particles through a base material
Phase transformations
Heated to form a single phase at an elevated temperature
TTT (Time-Temperature-Transformation) Diagram
assume that the properties of instantaneous heating followed by constant temperature transformation match reality
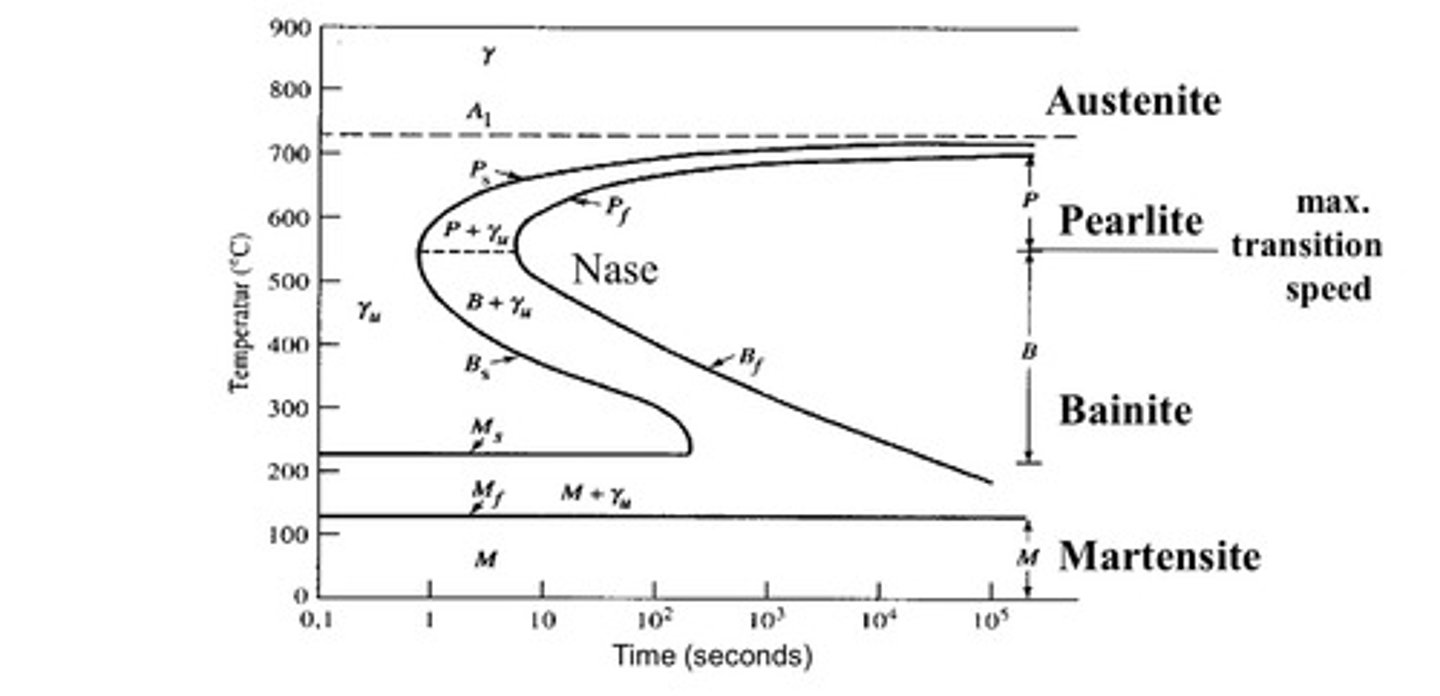
Martensite
very hard, strong, but brittle. lacks the toughness and ductility for engineering applications
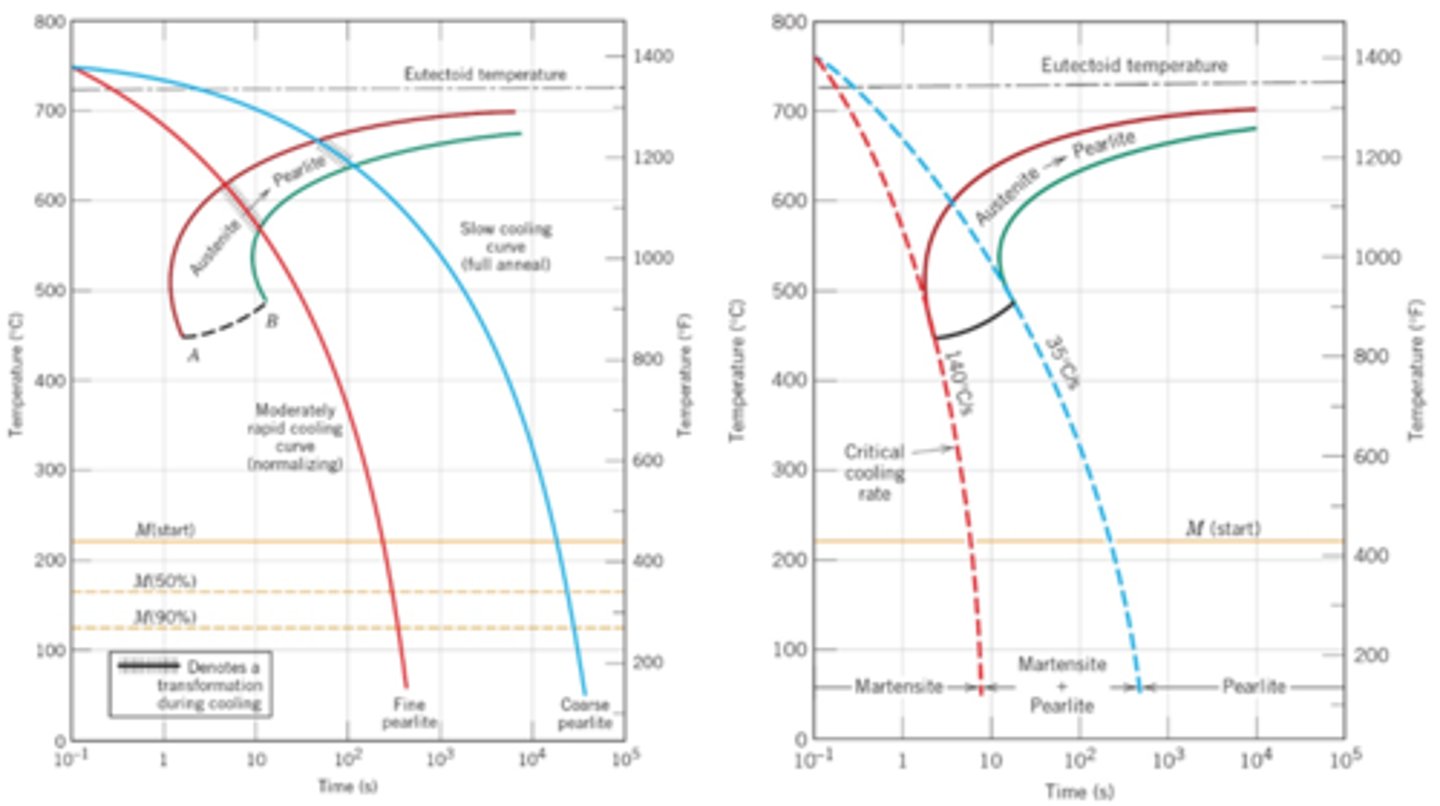
CCT Diagram
show a more accurate picture of the transformations
Jominy test
test used to evaluate hardenability, an austenitized steel bar is quenched atone end only thus producing a range of cooling rates
Hardenability Considerations
The greater a material's hardenability, the easier it is for a material to be slow cooled. Slow cooling reduces the probability of quench-cracking
Quenchants
he medium in which a material is quenched (rapid cooling of material at elevated temperature)
Formation of the vapor jacket
1st stage of quenching. Thin gaseous layer between the metal and the liquid during cooling
Nucleate boiling phase
2nd stage of quenching. Produces rapid rates of cooling down to the boiling point of the quenchant
Conduction and convection
3rd stage of quenching. Slower cooling from the boiling point to room temperature
Water
effective quenching medium because of its high heat of vaporization and relatively high boiling point
Brine (salt water)
Similar to water. Rapid cooling occurs because the salt nucleates bubbles. Corrosion problems may exist
Oil
utilized if slower quenching rates are desired. may cause water contamination, smoke, fumes, etc. More expensive than water or brine quenchants
Residual stresses
Stresses that exist in a part independent of an applied stress. Most parts being heat treated experience nonuniform temperatures during cooling or quenching can lead to this
Ways to prevent quench cracking and residual stresses
More uniform cross-sectional area
Generous fillets
Radiused corners
Smooth transitions
Adding additional holes
Ausforming
Material is heated to form austenite and then quenched to a temperature in the ``bay''between pearlite and bainite, maintaining the austenite. One may then deform the material, and then temper it to increase ductility.
case hardening
Methods to produce properties that vary throughout the material, i.e., hard surface, soft core
Flame hardening
Uses an oxy-acetylene flame to raise the surface temperature to reform austenite
Induction hardening
Steel part is placed inside a conductor coil and alternating current is used to change the surface of the steel . Rate and depth of heating can be controlled. Ideal for round bars and cylindrical parts
Laser beam hardening
Produces hardened surfaces . Absorptive coatings (zinc or manganese phosphate) are applied to the steel to increase efficiency
Electron beam hardening
Heat source is a beam of high-energy electrons
Carburizing
diffusion of carbon into FCC austenite steel at elevated temperatures
gas carburizing
hot gas containing carbon surround the part
pack carburizing
he steel is surrounded by a solid (charcoal for example) that contains carbon
liquid carburizing
the steel is placed in a molten bath with carbon
Nitriding
hardens the surfaces by producing alloy nitrides in special steels that contain nitride-forming elements typically heated in a dissociated ammonia
Ionitriding
plasma process that places parts in an evacuated furnace and treats them with direct current potential. Low pressure nitrogen is then introduced into the furnace and becomes ionized
Ion carburizing
similar to ionitriding except that methane is introduced instead of nitrogen
Carbonitriding
where both nitrogen and carbon are introduced
Steel
Mostly used by automotive industries
Plain Carbon Steel
Theoretically, steel is an alloy of only iron and carbon, but steel contains other elements in detectable amounts. Strength is primarily a function of carbon content
Low Carbon Steels
have less than 0.20% carbon and have good formability
Medium Carbon Steels
have between 0.20% and 0.50% carbon . Best balance of properties . High toughness and ductility are good with respect to the levels of strength and hardness
High carbon steels
have more than 0.50% carbon. Toughness and formability are low, but hardness and wear resistance are high
Carbon steels
have high strength, high stiffness, and reasonable toughness. Rust easily and require surface protection
Aluminum
Alloying element in nitriding steels
Bismuth
Improves machinability
Boron
Powerful hardenability agent
Chromium
Increase of hardenability, corrosion resistance,
Copper
Corrosion resistance, improved machinability
Lead
Improved machinability
Manganese
Prevents brittleness, increases hardenability
Molybdenum
Inhibits grain growth
Nickel
Toughener, Corrosion resistance
Silicon
Increases strength, spring steels
Sulfur
Free-machining properties
Titanium
Reduces martensitic harness in chromium stels
Tungsten
Hardness at high temperatures
Vanadium
Increases strength while retaining ductility, Prmotes fine grain structure
AISI-SAE Classification System
First number indicates the major alloying elements
Second number designates a subgrouping within the major alloy system
Last two digits indicate the carbon percentage
High-strength low-alloy (HSLA)
Provide increased strength to weight ratio
Modest increase in cost
Available in sheet, strip, plate, structural shapes, and bars
High yield strength, good weldability, and good corrosion resistance
Microalloyed Steels
are between carbon steels and alloy grades with respect to cost and performance
offer maximum strength with minimum carbon
Preserves weldability, machinability, and formability
Energy savings can be substantial
Advanced High-Strength Steels (AHSS)
Enable the stamping or hydroforming of complex parts
Higher strength provides improved fatigue resistance
Dual-phase steels can absorb more energy, meaning they are better for crash resistance in automotive applications
Free-Machining Steels
Machine readily and form small chips when cut
The smaller the chips reduce friction on the cutting tool which reduces the amount of energy required (reduces tool wear)
Maraging steels
Used when extremely high strength is required
Typically also have high toughness
Steels for High-Temperature Service
Plain-carbon steels should not be used for temperatures in excess of 250°C
Tend to be low-carbon materials
Ferritic stainless steel
Limited ductility, Poor toughness, Readily weldable, Cheapest
Martensitic stainless steels
Increased strength, More carbon content, less chromium , Less corrosion resistant but more expensive than ferritic
Water-hardening tool steels (W)
Least expensive method for small parts that are not subjected to extreme temperatures
Cold-work steels (O,A)
Larger parts that must be hardened
Oil or air quenched grades
Shock resisting tool steels (S)
Offers high toughness for impact applications
High speed tool steels
Used for cutting tools where strength and hardness are needed at high temperatures
Hot-work steels (H)
Provide strength and hardness during high temperature applications
Plastic mold steels (P)
Meets requirements of zinc die and plastic injection molding
Special purpose tool steels (L,F)
Extreme toughness, extreme wear resistance
Casting process
Material is melted
Heated to proper temperature
Treated to modify its chemical makeup
Solidifies
Advantages of Casting
Complex shapes, hollow sections or cavities, large parts, mold materials
Parting line
Separates the cope and drag
Draft
Angle or taper on a pattern that allows for easy removal of the casting from the mold
Casting
Process and product when molten metal is poured and solidified
Casting Defects
Gas porosity, Shrinkage
Chill Zone
Rapid nucleation that occurs when the molten metal comes
into contact with the cold walls of the mold
Columnar Zone
Rapid growth perpendicular to the casting surface
Equiaxed Zone
crystals in the interior of the casting, spherical randomly oriented crystals
Shrinkage
Shrinkage of liquid as it
cools from the solidification
temperature
Solidification shrinkage as
the liquid turns into solid
Solid metal contraction as
the solidified metal cools to
room temperature
Risers
Reservoirs of liquid metal that feed extra metal to the mold to compensate for shrinkage
Blind Riser
contained entirely within the mold cavity
Live Riser
Receive the last hot metal that enters the mold and generally do so at a time when the metal in the mold cavity has already begun to solidify.
Sand Casting
a metal casting process characterized by using sand as the mold material.
Requirements of sand used in casting
Refractoriness
Cohesiveness
Permeability
Collapsability
Dump-Box Shell Molding
Flip
Complex internal cavities can be produced with
cores
Die Casting
Similar to permanent mold casting except that the metal is injected into the mold under high pressure.
Centrifugal Casting
inertial forces of rotation or spinning are used to distribute the molten metal into the mold cavity
Powder metallurgy
Metal processing technology in which parts are produced from metallic powders
Basic Process of Powder Metallurgy
Powder manufacture
Mixing or blending
Compacting
Sintering
Isostatic compaction
when extremely complex shapes are desired, the powder is generally encapsulated in a flexible mold and immersed in a pressurized gas or liquid process
Sintering
a process whereby particles are heated to the point that they fuse together at their borders but do not clump into one solid mass