Chapter 3 & 5: Bioenergetics, Enzymes and Metabolism
What is the difference between anabolism and catabolism
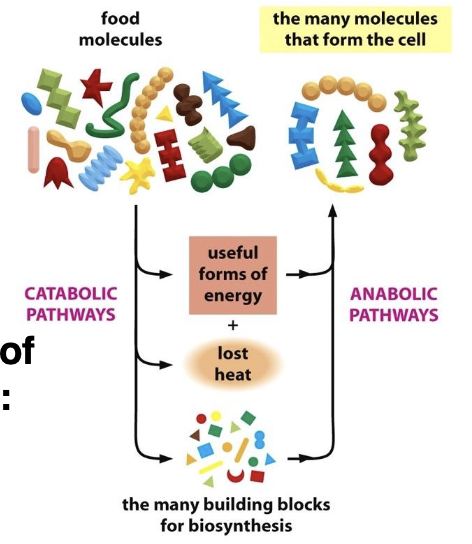
Anabolism refers to the metabolic pathways that construct large molecules from smaller units, requiring energy input
catabolism involves breaking down large molecules into smaller units, releasing energy in the process.
What is Metabolism
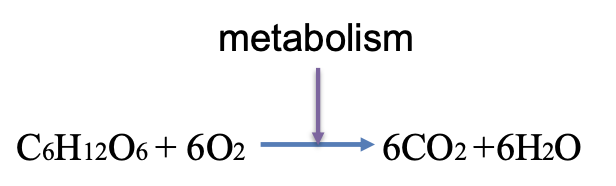
all the chemical processes going on continuously inside your body that allow life and normal functioning (homeostasis)
two types: Catabolism and Anabolism
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
What is the difference between anabolism and catabolism
Anabolism refers to the metabolic pathways that construct large molecules from smaller units, requiring energy input
catabolism involves breaking down large molecules into smaller units, releasing energy in the process.

What is Metabolism
all the chemical processes going on continuously inside your body that allow life and normal functioning (homeostasis)
two types: Catabolism and Anabolism
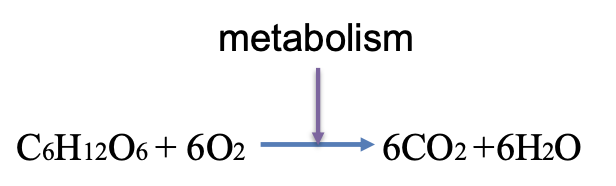
What’s the basic principles of the oxidation states of carbon
The oxidation states of carbon indicate its degree of oxidation or reduction (moving electrons/energy), reflecting how many electrons it has gained or lost.
These states can influence the stability and reactivity of organic compounds
Five oxidation states of carbon (depending on the elements with which carbon shares electrons)
Lots of steps to get alkane to carbon dioxide – the end goal
Carbon dioxide is the most highly oxidized form of carbon found in living organisms
What are the characteristics of exergonic and endergonic rxns and rxn equilibrium
Exergonic reactions release energy and occur spontaneously
∆G (energy associated with a chemical reaction) is negative
Endergonic reactions absorb energy and are non-spontaneous, ex. glycolysis
∆G is positive
Reaction equilibrium is the point at which the rate of forward and reverse reactions are equal, resulting in stable concentrations of reactants and products.
∆G is 0
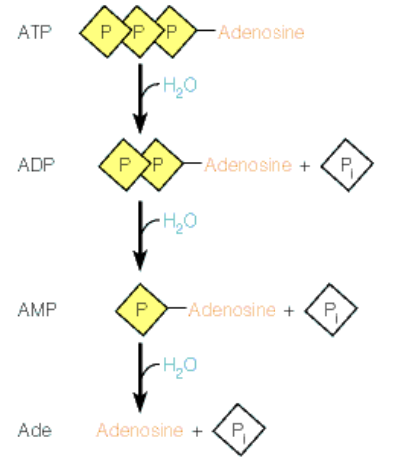
What is free energy changes of ATP
The free energy changes of ATP refer to the energy released when ATP is hydrolyzed to ADP and inorganic phosphate, driving various biochemical processes
This energy change is crucial for cellular work, facilitating reactions that require energy input
Hydrolysis: breaking bonds apart using water
Explain the concept of coupling rxns
In metabolism, pathways (like glycolysis) must be thermodynamically favorable but not each reaction needs to be thermodynamically favorable because reactions can be coupled – “sharing” potential transfer energy when they share reaction intermediates
Coupling reactions involve linking an exergonic reaction to an endergonic reaction, allowing the energy released from the former to drive the latter.

How would you ‘activate a molecule of glucose in the first step of glycolysis by coupling a reaction (including the names of substrate enzyme and product)
Glucose+ATP→Glucose-6-phosphate (G6P)+ADP
In glycolysis, glucose is phosphorylated to glucose-6-phosphate by the enzyme hexokinase (or glucokinase in liver cells), using ATP as the substrate, which is converted to ADP.
substrates: glucose and atp
products: glucose-6-phosphate (G6P)
this reaction couples the energy released from ATP hydrolysis to activate glucose for further metabolism down the glycolysis pathway
this is an irreversible step

What is the difference between ∆G˚ and ∆G
∆G˚ represents the standard free energy change under standard conditions
change in free energy during a reaction under standard conditions and measured in a test tube
∆G indicates the actual free energy change under specific conditions of concentration and temperature
changes in free energy associated with a chemical reaction in a cell
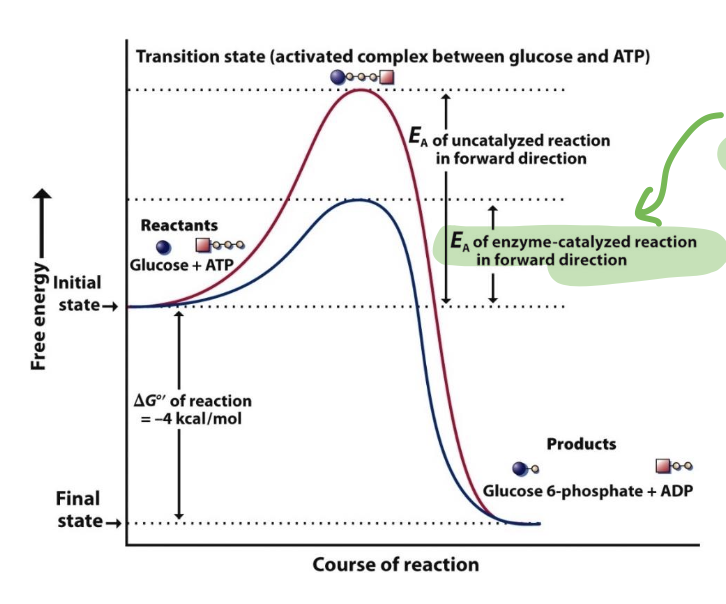
What’s the role of a catalyst and how it increases the rate of a rxn
A catalyst is a substance that accelerates the rate of a chemical reaction without being consumed in the reaction itself.
It lowers the activation energy required for the reaction to proceed (the transition substrate), allowing reactants to convert to products more efficiently.
only affects the rates of reactions
Enzymes (biological catalysts) are the mediators of metabolism, responsible for virtually every reaction that occurs in a cell.
Without enzymes, metabolic reactions would proceed so slowly as to be imperceptible.
very specific
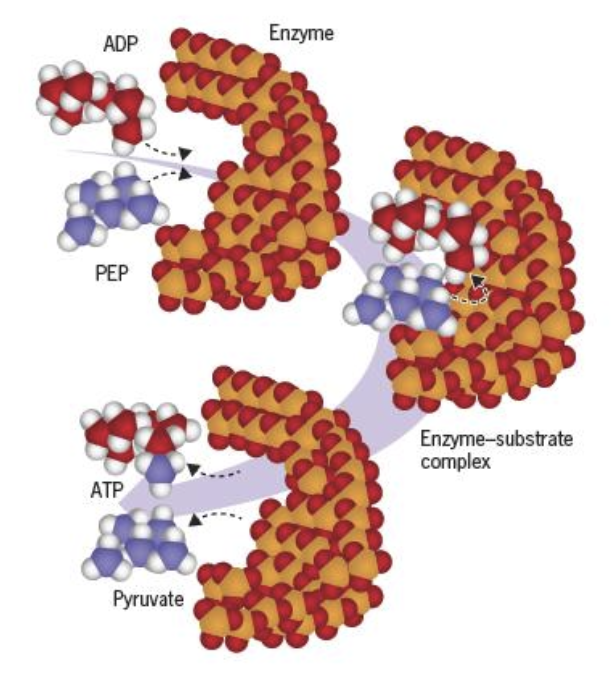
What is a active site
The active site is a region on an enzyme where substrate molecules bind, forming an enzyme-substrate complex.
It contains specific amino acids that facilitate the conversion of substrates to products through a precise fit and induced conformation changes.
The active site and the substrate have complementary shapes that allow substrate specificity but after binding of substrate to enzyme both will change conformation
binding via multiple weak non-covalent interactions
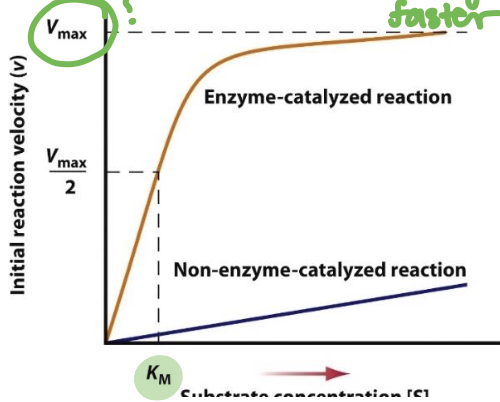
What is meant by maximal velocity
Maximal velocity, or Vmax, refers to the maximum rate at which an enzyme-catalyzed reaction can proceed when the enzyme is saturated with substrate.
It indicates the efficiency and capacity of the enzyme under optimal conditions.
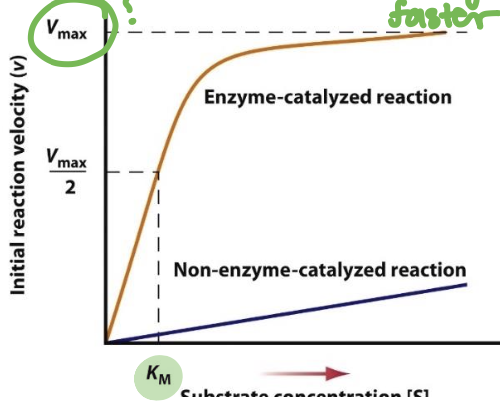
What is meant by Km
Km, or Michaelis constant, is the substrate concentration at which an enzyme-catalyzed reaction reaches half of its maximal velocity (Vmax)
It reflects the affinity of the enzyme for its substrate; a lower Km indicates higher affinity.
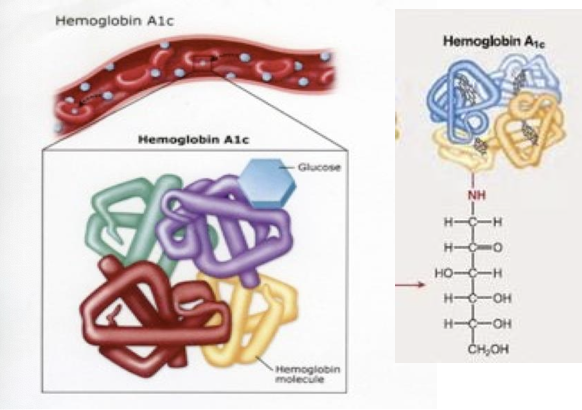
What is glycated hemoglobin and how can it be used to determine average blood glucose levels over a period of time
Glycated hemoglobin (GHB), or A1c, is a form of hemoglobin that is chemically linked to glucose
Is used to measure “average” blood glucose concentrations over time (over the previous two months) making it a crucial marker for diagnosing and managing diabetes
when you have excess glucose it then forms complexes, which causes a lot of problems
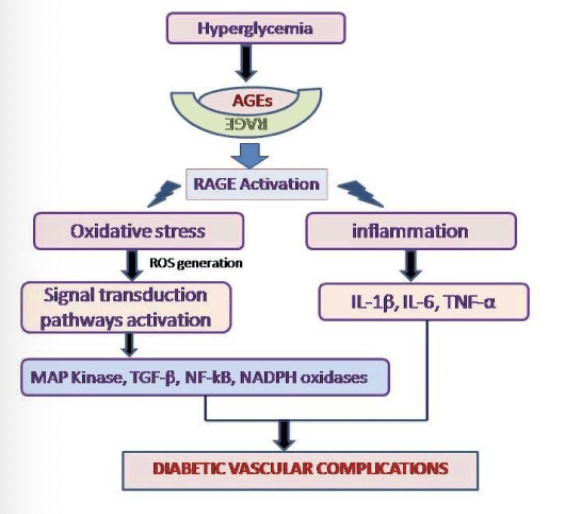
What’s the definition of AGE with respect to glycated proteins
AGE, or Advanced Glycation End-products, are harmful compounds formed when proteins or lipids (not just hemoglobin) bond with sugars, which can accumulate and contribute to various chronic diseases, including diabetes and cardiovascular issues.
The more of this that occurs, the higher the potential damage
What are inhibitors and explain how it being covalent or not affects it?
Inhibitors are substances that decrease the activity of enzymes by binding to them
Irreversible inhibitors bind tightly to the enzyme → permanent (often covalently)
Reversible inhibitors bind loosely to the enzyme → temporary (non covalently)
What is competitive inhibition
Reversible inhibition
Is a process where a substance similar to the substrate competes for binding to the active site of an enzyme, reducing the enzyme's activity and slowing down the reaction rate.
usually resemble the substrate in structure but high enough levels of substrate can compete out the inhibitor
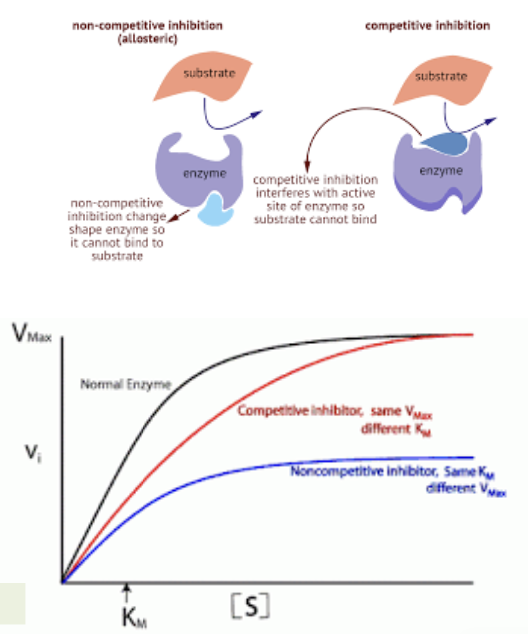
What is non-competitive inhibition
Reversible inhibition
Is a type of enzyme inhibition where an inhibitor binds to an enzyme at a site other than the active site, altering the enzyme's shape and function (conformation), thus decreasing its activity regardless of substrate concentration.
Allosteric inhibitors
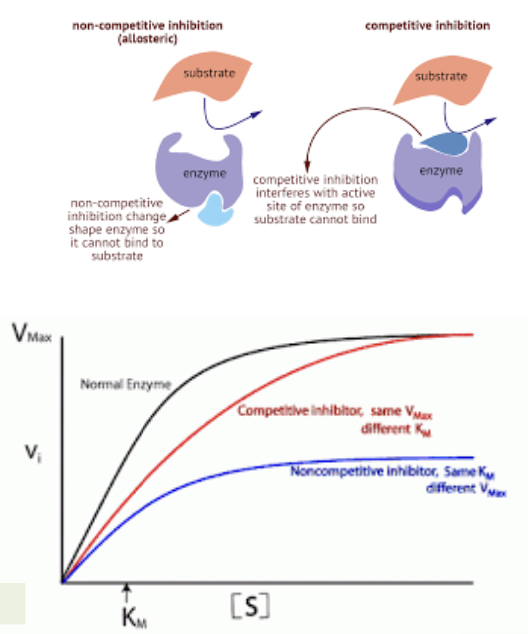
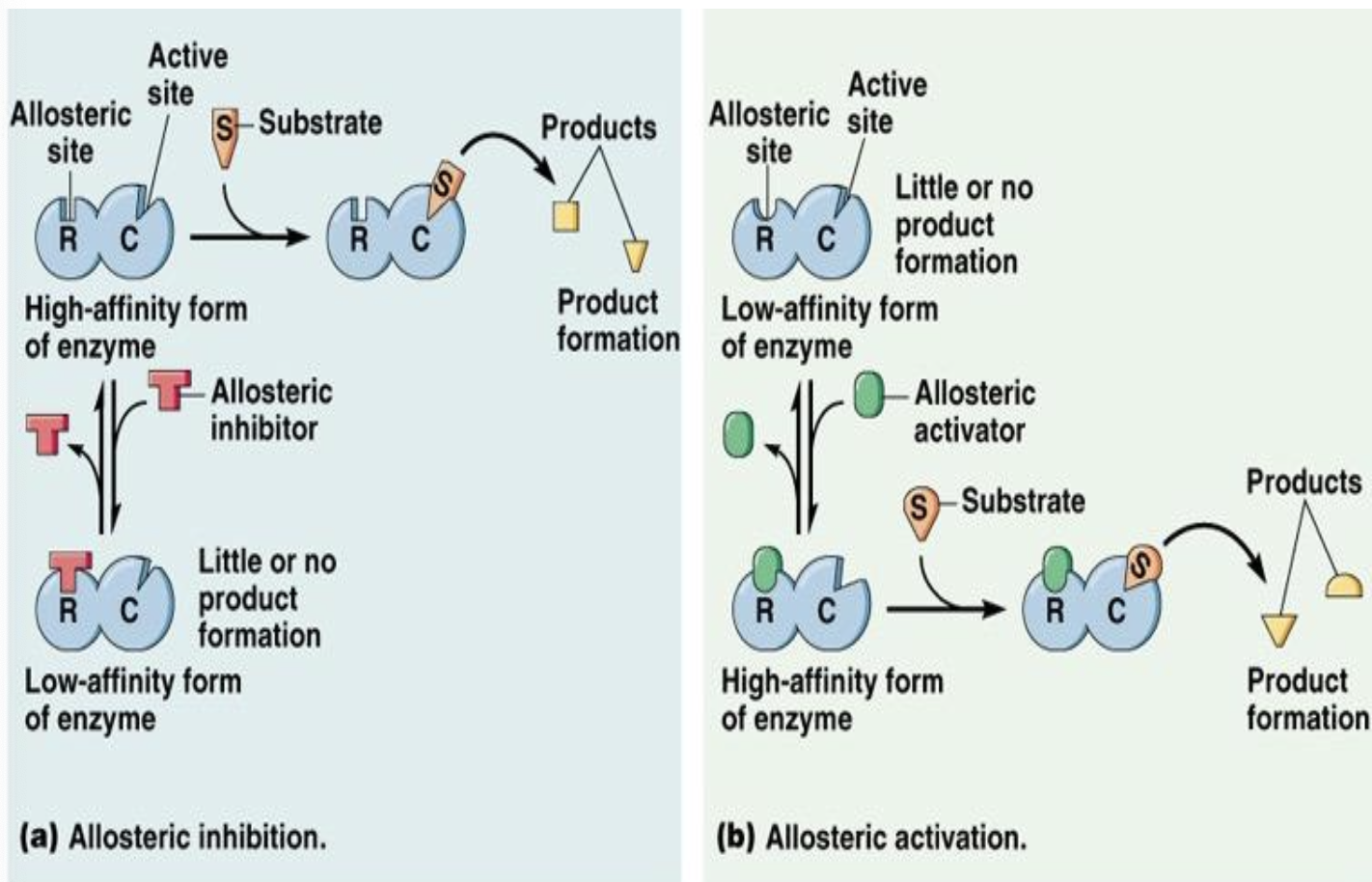
What does allosteric inhibition mean
Allosteric inhibition refers to a regulatory mechanism where an inhibitor binds to an allosteric site on an enzyme, causing a conformational change that decreases the enzyme's activity and prevents substrate binding.
Allosteric modulation of enzymes can inhibit or activate enzyme activity
PKA (protein kinase A) is activated by catalytic subunits and cAMP
What is the role of NADH and FADH2
NADH and FADH2 are coenzymes that function as electron carriers in cellular respiration, transferring electrons to the electron transport chain to produce ATP during oxidative phosphorylation
all of this takes place in the mitochondrial matrix and the citric acid cycle, hence is why we are generating so many of these electron carriers
NADH carries 2 electrons that will be passed on to the electron transport chain and will ultimately be transferred to molecular oxygen which ultimately results in the formation of ATP
FADH2 also carries 2 electrons that follow the same pathway
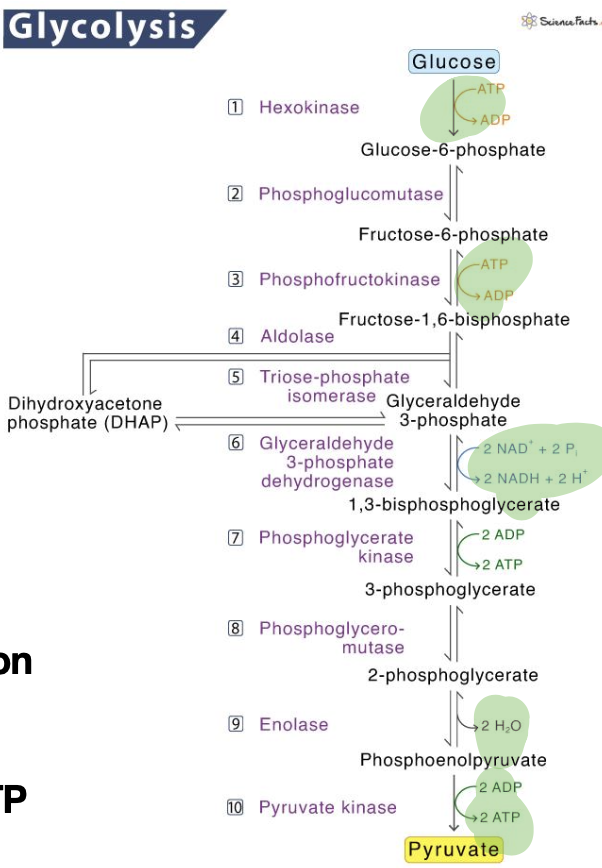
What happens to the carbons from glucose when glucose is used to generate ATP
The carbons from glucose are converted into carbon dioxide through the processes of glycolysis, the citric acid cycle, and the subsequent oxidation of pyruvate.
This release of carbon dioxide occurs as glucose is metabolized for energy
conserved energy is used to pump protons from matrix to intermembrane space where it ultimately leads to atp being made (down the transport chain)
Ultimately, carbons get excreted from glucose
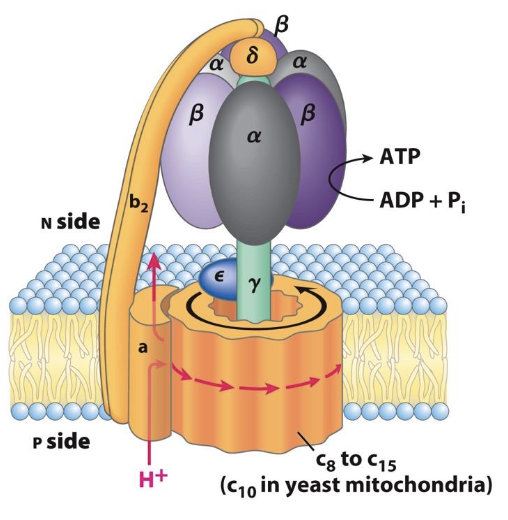
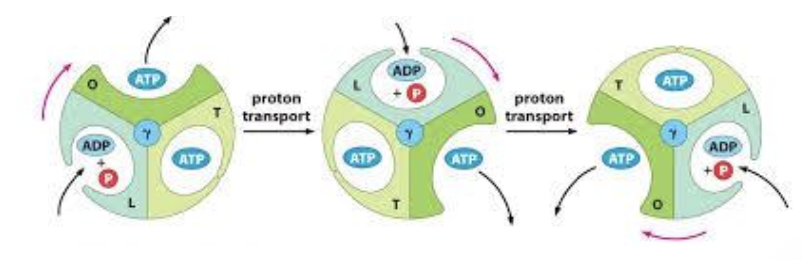
What are the 3 conformations of ATP synthase and what happens at each step (that is the synthesis of ATP)
know the 3 subunits (alpha and beta x3) which change conformations, substrates involved (ADP) – moving 120 degrees resulting in ATP – moving another 120 degrees will release the ATP
ATP synthase has three conformations: open (O), loose (L), and tight (T).
In the open conformation, ADP and inorganic phosphate enter
In the loose conformation, they are held together
In the tight conformation, ATP is synthesized, which is then released back into the mitochondrial matrix.