Sterile Compounding Module 2
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
What should you do prior to entering the buffer area?
- remove all outer garments
- remove all cosmetics
- remove all visible jewelry or piercings
- no artificial nails
- nails neat and trimmed, no polish
Does garbing protect the preparation from the operator or protect the operator from the preparation?
both
Garb from most _________ to most ___________
dirty;clean
This is 15% of all flora, and is removed with hand washing
transient hand flora
85% of all flora, not removed by hand washing
resident hand flora
What requires physical removal with hand washing, it is not removed with hand sanitizers or alcohol?
spores such as C. diff
Wash hands for at least ________
30 seconds
_________ sites are at greatest risk of contamination
- touch
- moisture
- contact with unclean air
critical sites
ALL critical sites must be in contact with _______________ AT ALL TIMES during compounding
first air
What are the critical sites of a needle? (select all)
A. hub
B. tip
C. shaft
D. cap
A. hub
B. tip
C. shaft
What are the critical sites of a syringe? (select all)
A. ribs of the plunger
B. tip
C. shaft
D. cap
A. ribs of the plunger
B. tip
What are the critical sites of vials and ampules? (select all)
A. ribs of the plunger
B. tip
C. rubber closure when penetrated
D. opening of ampule
C. rubber closure when penetrated
D. opening of ampule
Wipe down supplies prior/after placing in PEC
prior
- Swab vials, ampules, and additive ports with sterile, lint-free alcohol wipes
- Use clean alcohol swab for each product
- allow to remain wet for at least ______ seconds
10
- Remove syringe from package then pull needle wrapper back and attach needle to syringe
- Do/Do not push through outer wrapper
- Remove cap using push-pull technique
- open syringe at least ____ inches inside hood
- do not
- 6
- syringes should be capped when medications will be administered directly from the syringe
- should not be sent with needle attached
- store caps in ISO _____ apartment
5
What type of pressure is this?
- air added exceeds volume of solution withdrawn
positive pressure
How do you remove positive pressure?
remove by allowing air to expel from vial into syringe until plunger stops moving. Withdraw a bit more air then remove syringe from vial. Should equilibriate pressure
What type of pressure is this?
- if amount of air removed exceeds volume of solution removed
- results in difficulty removing volume needed from vial
- preferred when working with hazardous agents
negative pressure
What are the components of a label?
- patient name
- patient room number
- additional patient identifiers (medical record number or encounter number)
- names and concentrations of all ingredients
- volumes of all ingredients
- total volume of preparation
- instructions for administration
- route and rate of admin
- date of prep
- must specify that it is a compounded product
- BUD
- preparer's initials
- pharmacist's initials
What is this describing:
- involves the compounding of multiple preparations containing the same ingredients
- prepared in one extended process
- requires specific documentation (including master formula sheet)
- may be used for compounding for up to 12 hours
batch compounding
USP __________
-guidelines for sterile preparation
797
USP ___________
- describes practice and quality standards for handling hazardous drugs to promote patient safety, worker safety, and environmental protection
800
What are the categories that a hazardous drug can fall into?
- carcinogenicity
- teratogenicity or developmental toxicity
- organotoxicity at low doses in humans
- reproductive toxicity in humans
- genotoxicity
Who decides what drugs are hazardous?
NIOSH
- National Institute for Occupational Safety and Health
What drugs on the NIOSH list MUST FOLLOW the requirements of USP 800?
- any hazardous drug active pharmaceutical ingredient
- any antineoplastic requiring HD manipulation
What drugs on the NIOSH DO NOT HAVE TO FOLLOW all the containment requirements of this chapter if an assessment of risk is performed and implemented:
- final dosage forms... including antineoplastic dosage forms that do not require any further manipulation other than counting or repackaging (unless required by the manufacturer)
What does an assessment of risk look like?
- type of HD
- dosage form
- risk of exposure
- packaging
- manipulation
- alternative containment strategies and/or work practices
- must be reviewed every 12 months
What is the process of receiving hazardous drugs?
- receipt takes place in a segregated area of a negative pressure room
- deliver is in labeled hard sided container
- drugs are packaged in closed leakproof bags
- they are wiped down with bleach
- transported in another hard sided container to pharmacy
- HDs must NOT be unpacked from their external shipping containers in sterile compounding areas or in positive pressure areas
know this
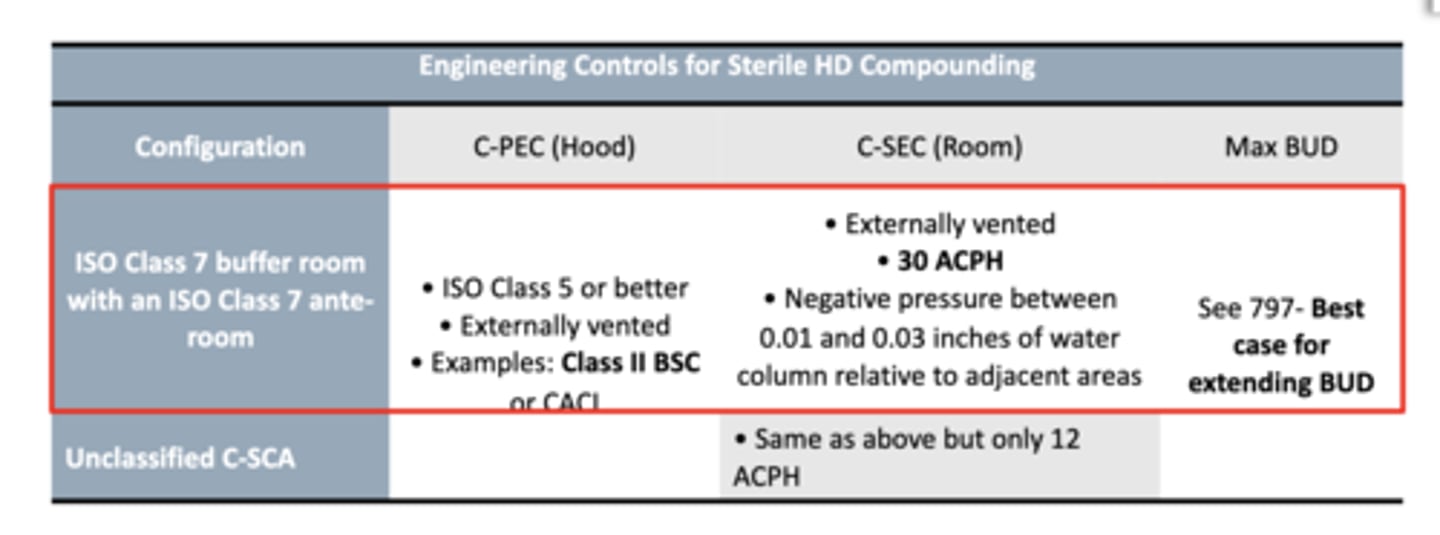
What is this describing:
- prohibits the transfer of environmental contaminants into the system and the escape of HD or vapor concentrations outside the system
- aim to reduce surface contamination
- reduce or eliminate the likelihood of needle sticks
close system transfer device
This is the purpose of what cleaning step:
- render compound inert or inactive
- Ex: EPA-registered oxidizers (peroxides, sodium hypochlorite)
deactivation
This is the purpose of what cleaning step:
- remove HD residue
- Ex: EPA-registered oxidizers (peroxides, sodium hypochlorite), alcohols, H2O
decontamination
This is the purpose of what cleaning step:
- remove organic and inorganic material
- Ex: germicidal detergent
cleaning
This is the purpose of what cleaning step:
- destroy microorganisms
- EPA-registered disinfectant and/or sterile alcohol
disinfection
Is this a major or minor spill?
- <5 mLs contained in the PEC may be handled by trained pharmacy staff
minor spill
Is this a major or minor spill?
- >5 mLs must be managed according to policy and will require a spill kit
major spill
When do the SOPs need to be reviewed by the designated person
every 12 months at least